A kind of ganglioside sustained-release tablet and preparation method thereof
A technology for ganglioside and sustained-release tablets, which is applied in nervous system diseases, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of inconvenient use, non-standard production, high price, etc. High, low price, low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
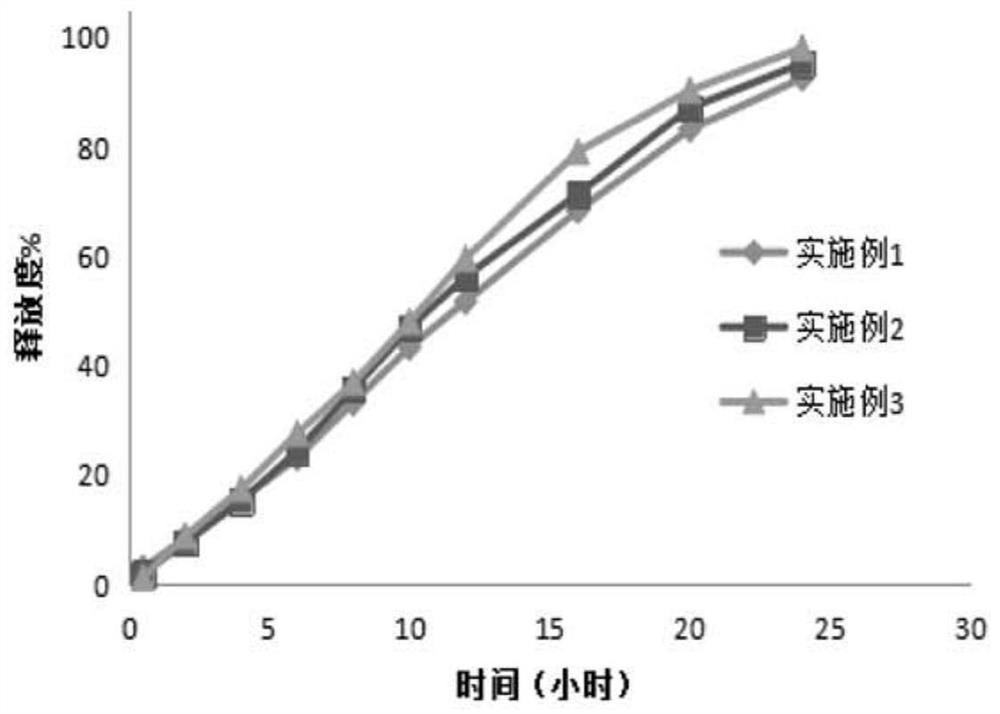
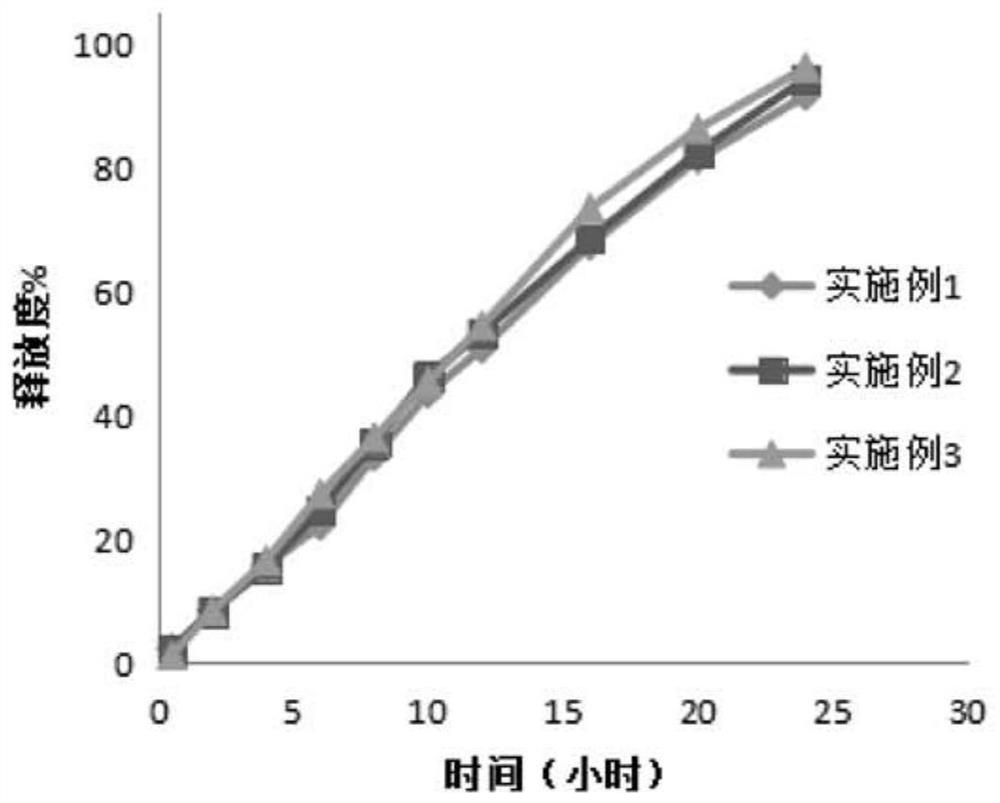
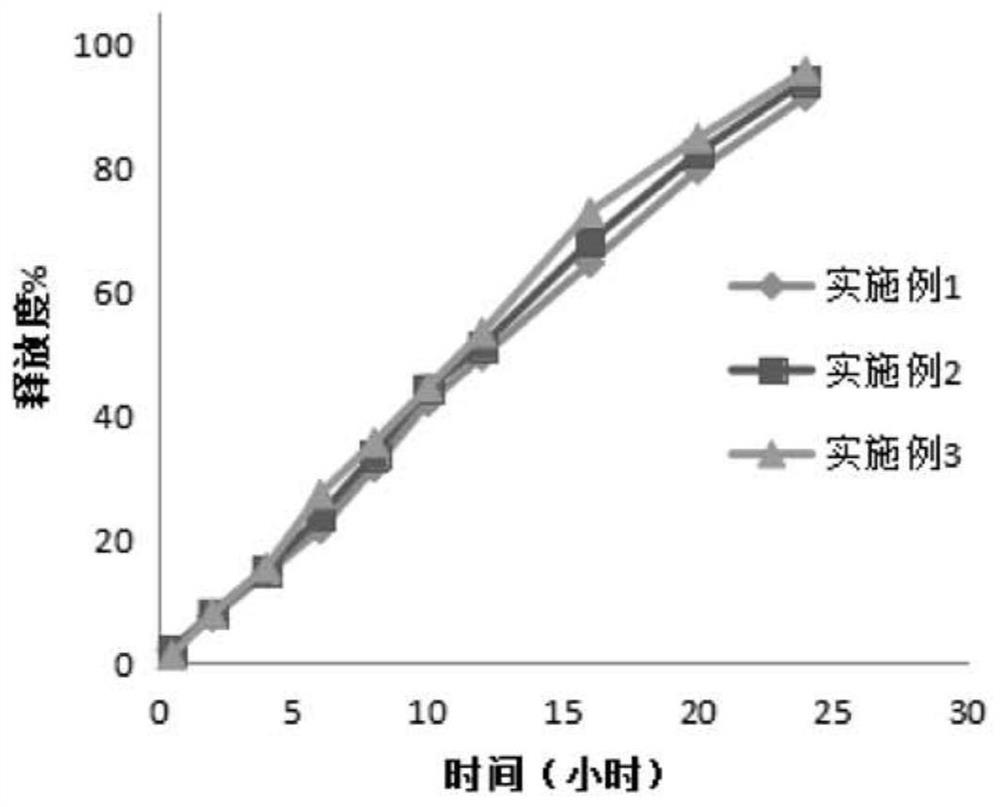
Examples
Embodiment 1
[0034] Preparation of Example 1 Ganglioside Sustained-release Tablets (300mg / tablet, 1000 tablets were prepared):
[0035] Tablet prescription:
[0036] Element Dosage content Sodium Monosialotetrahexosylganglioside 55g 16.77% Ethyl cellulose 180g 54.88% Hypromellose Phthalate 45g 13.72% arginine 12g 3.66% povidone 13g 3.96% lactose 20g 6.10% Magnesium stearate 3g 0.91% gross weight 328g 100%
[0037] Coating Solution Prescription:
[0038] Element Dosage content Hypromellose 20g 80% polyethylene glycol 6000 2g 8% talcum powder 2g 8% Iron Oxide Red 1g 4% Total 25g 100%
[0039] Sustained-release tablet preparation process:
[0040] (1) Preparation of tablet core: pulverize monosialotetrahexosylganglioside sodium, lactose, ethyl cellulose, and arginine, pass through a 100-mesh sieve, mix mechanically, and mix 5% povidone Spray the aqueous solution in...
Embodiment 2
[0042] The preparation of embodiment 2 ganglioside sustained-release tablets
[0043] Tablet prescription:
[0044]
[0045]
[0046] Coating Solution Prescription:
[0047] Element Dosage content Hypromellose 20g 80% polyethylene glycol 6000 2g 8% talcum powder 2g 8% Iron Oxide Red 1g 4% Total 25g 100%
[0048] making process:
[0049] (1) Preparation of tablet core: pulverize monosialotetrahexosylganglioside sodium, lactose, ethyl cellulose, and arginine, pass through a 100-mesh sieve, mix mechanically, and mix 5% povidone Spray the aqueous solution into the mixture for 40-mesh sieve granulation. After granulation, spray 8% hypromellose phthalate ethanol solution to coat the granules, and dry at 60°C until the moisture content is 1.8%. , add magnesium stearate to the above dry granules, sieve through a 20-mesh sieve, place the granules in a granulator and mix evenly, the height of the upper punch is 14.8mm, and the ...
Embodiment 3
[0051] The preparation of embodiment 3 ganglioside sustained-release tablets
[0052] Tablet prescription:
[0053] Element Dosage content Sodium Monosialotetrahexosylganglioside 55g 16.67% Ethyl cellulose 170g 51.52% Hypromellose Phthalate 55g 16.67% arginine 10g 3.03% povidone 15g 4.55% lactose 22g 6.67% Magnesium stearate 3g 0.91% gross weight 330g 100%
[0054] Coating Solution Prescription:
[0055] Element Dosage content Hypromellose 20g 80% polyethylene glycol 6000 2g 8% talcum powder 2g 8% Iron Oxide Red 1g 4% Total 25g 100%
[0056] making process:
[0057] (1) Preparation of tablet core: pulverize monosialotetrahexosylganglioside sodium, lactose, ethyl cellulose, and arginine, pass through a 100-mesh sieve, mix mechanically, and mix 5% povidone Spray the aqueous solution into the mixture for 40-mesh sieve granulation. After granulation, sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com