Ganglioside sustained release tablet and preparation method thereof
A ganglioside and slow-release tablet technology, which is applied in nervous system diseases, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as inconvenient use, irregular production, single supply, etc., and achieve safety High, low price, low raw material cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
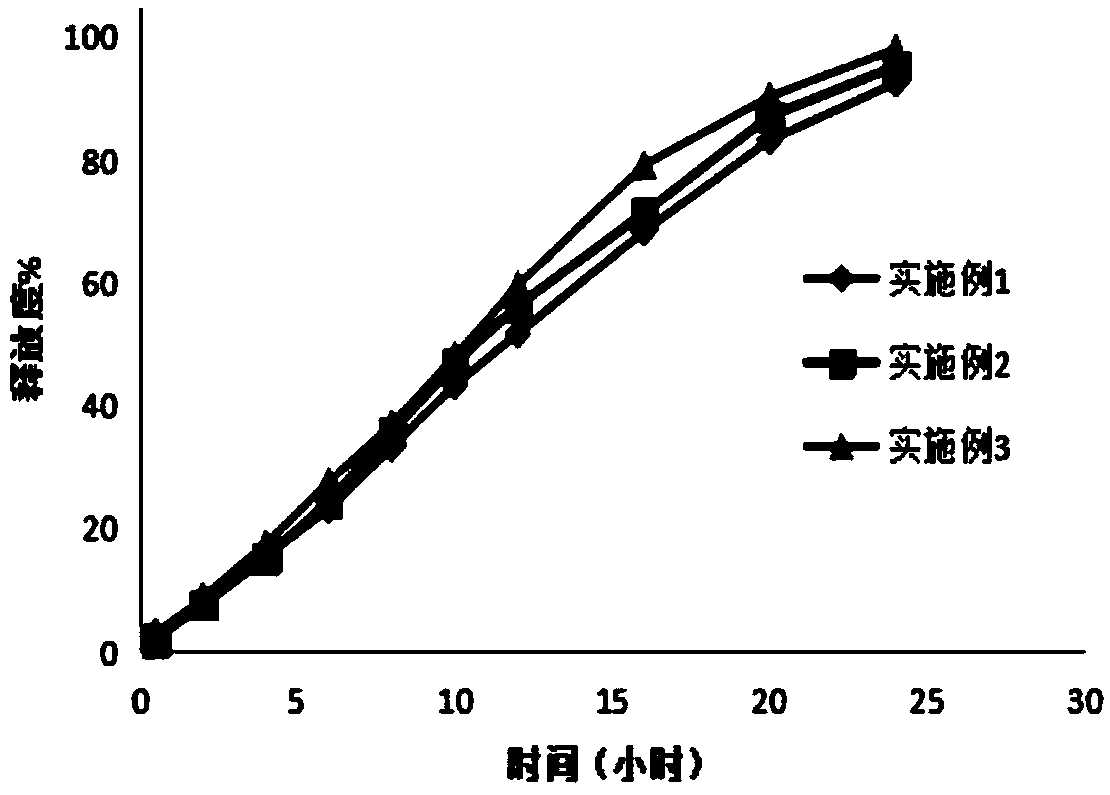
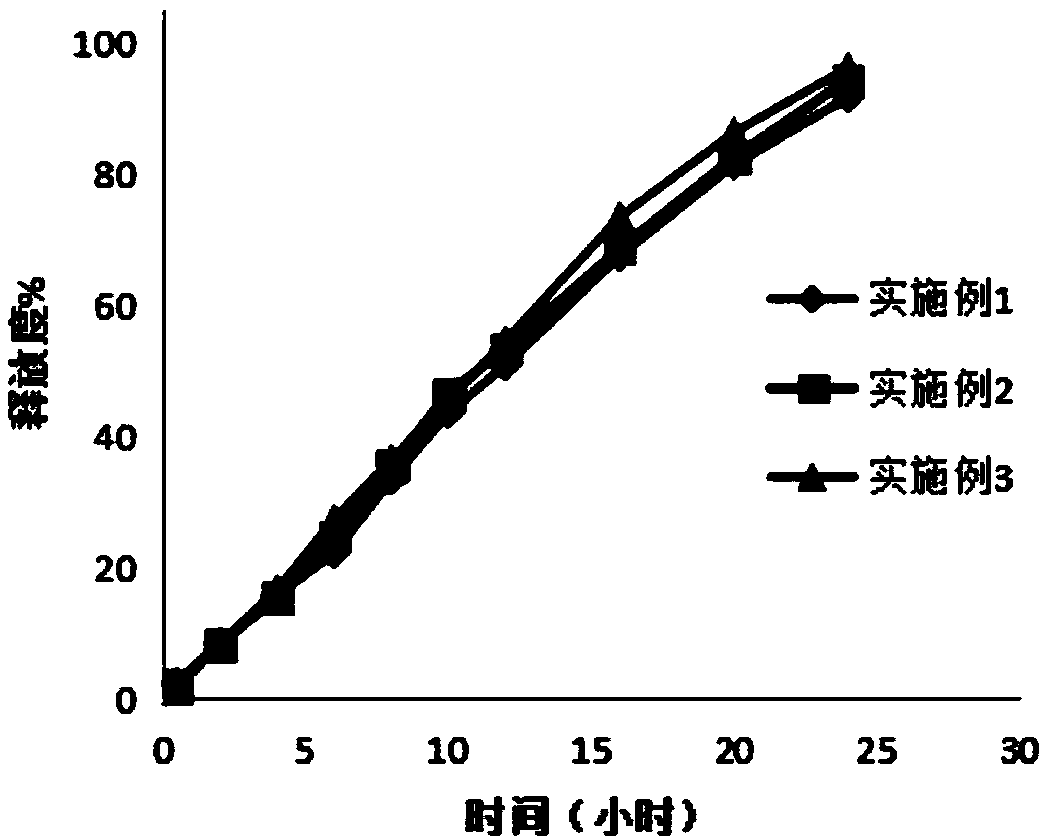
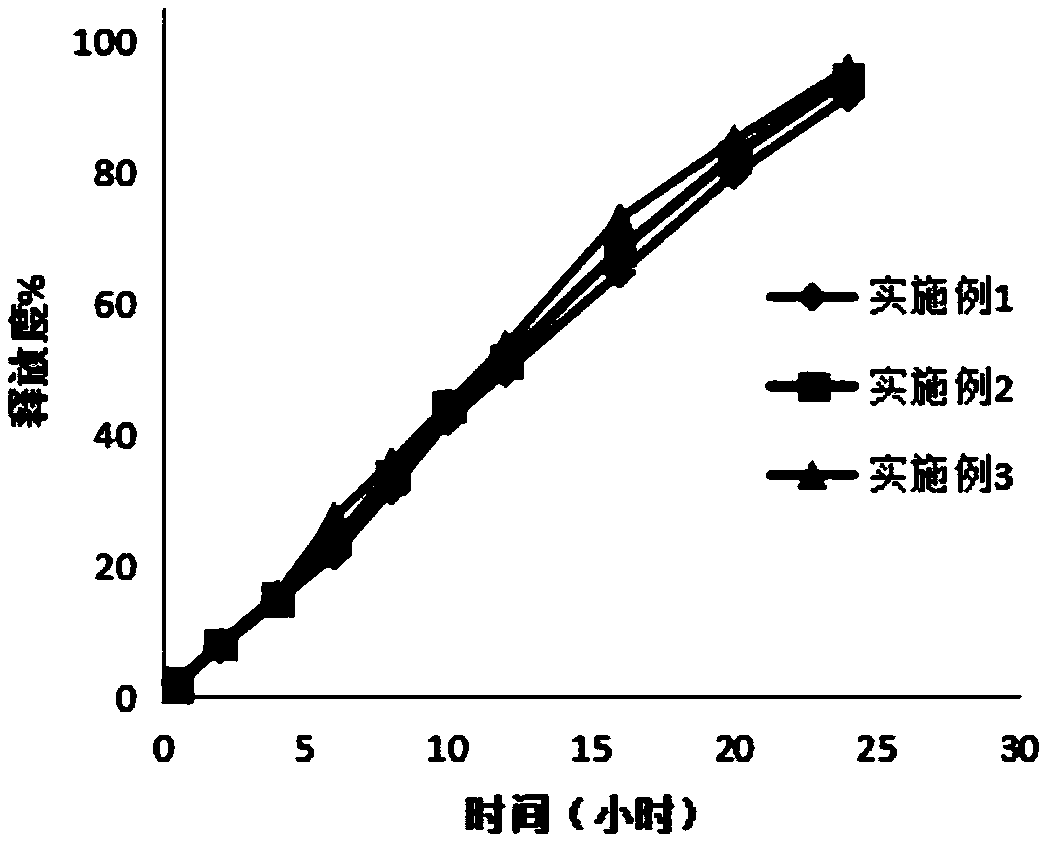
Image
Examples
Embodiment 1
[0034] Preparation of Example 1 Ganglioside Sustained-release Tablets (300mg / tablet, 1000 tablets were prepared):
[0035] Tablet prescription:
[0036] Element
Dosage
content
Sodium Monosialotetrahexosylganglioside
55g
16.77%
180g
54.88%
45g
13.72%
12g
3.66%
Povidone
13g
3.96%
20g
6.10%
3g
0.91%
gross weight
328g
100%
[0037] Coating Solution Prescription:
[0038] Element
Dosage
content
20g
80%
polyethylene glycol 6000
2g
8%
talcum powder
2g
8%
Iron Oxide Red
1g
4%
Total
25g
100%
[0039] Sustained-release tablet preparation process:
[0040] (1) Preparation of tablet core: pulverize monosialotetrahexosylganglioside sodium, lactose, ethyl cellulose, and arginine, pass through ...
Embodiment 2
[0042] The preparation of embodiment 2 ganglioside sustained-release tablets
[0043] Tablet prescription:
[0044]
[0045]
[0046] Coating Solution Prescription:
[0047] Element
Dosage
content
20g
80%
polyethylene glycol 6000
2g
8%
talcum powder
2g
8%
Iron Oxide Red
1g
4%
Total
25g
100%
[0048] making process:
[0049] (1) Preparation of tablet core: pulverize monosialotetrahexosylganglioside sodium, lactose, ethyl cellulose, and arginine, pass through a 100-mesh sieve, mix mechanically, and mix 5% povidone Spray the aqueous solution into the mixture for 40-mesh sieve granulation. After granulation, spray 8% hypromellose phthalate ethanol solution to coat the granules, and dry at 60°C until the moisture content is 1.8%. , add magnesium stearate to the above dry granules, sieve through a 20-mesh sieve, place the granules in a granulator and mix evenly, the height of t...
Embodiment 3
[0051] The preparation of embodiment 3 ganglioside sustained-release tablets
[0052] Tablet prescription:
[0053] Element
Dosage
content
Sodium Monosialotetrahexosylganglioside
55g
16.67%
170g
51.52%
55g
16.67%
arginine
10g
3.03%
Povidone
15g
4.55%
lactose
22g
6.67%
3g
0.91%
gross weight
330g
100%
[0054] Coating Solution Prescription:
[0055] Element
Dosage
content
Hypromellose
20g
80%
polyethylene glycol 6000
2g
8%
talcum powder
2g
8%
Iron Oxide Red
1g
4%
Total
25g
100%
[0056] making process:
[0057] (1) Preparation of tablet core: pulverize monosialotetrahexosylganglioside sodium, lactose, ethyl cellulose, and arginine, pass through a 100-mesh sieve, mix mechanically, and mix 5% povidone Spray the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com