Quality analysis method of Thesium chinense tablets
A quality analysis technology of Bairui tablets, applied in the field of quality analysis of Bairui tablets, can solve the problems of limited quality control of Bairui tablets and achieve remarkable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Determination method of Bairui tablet
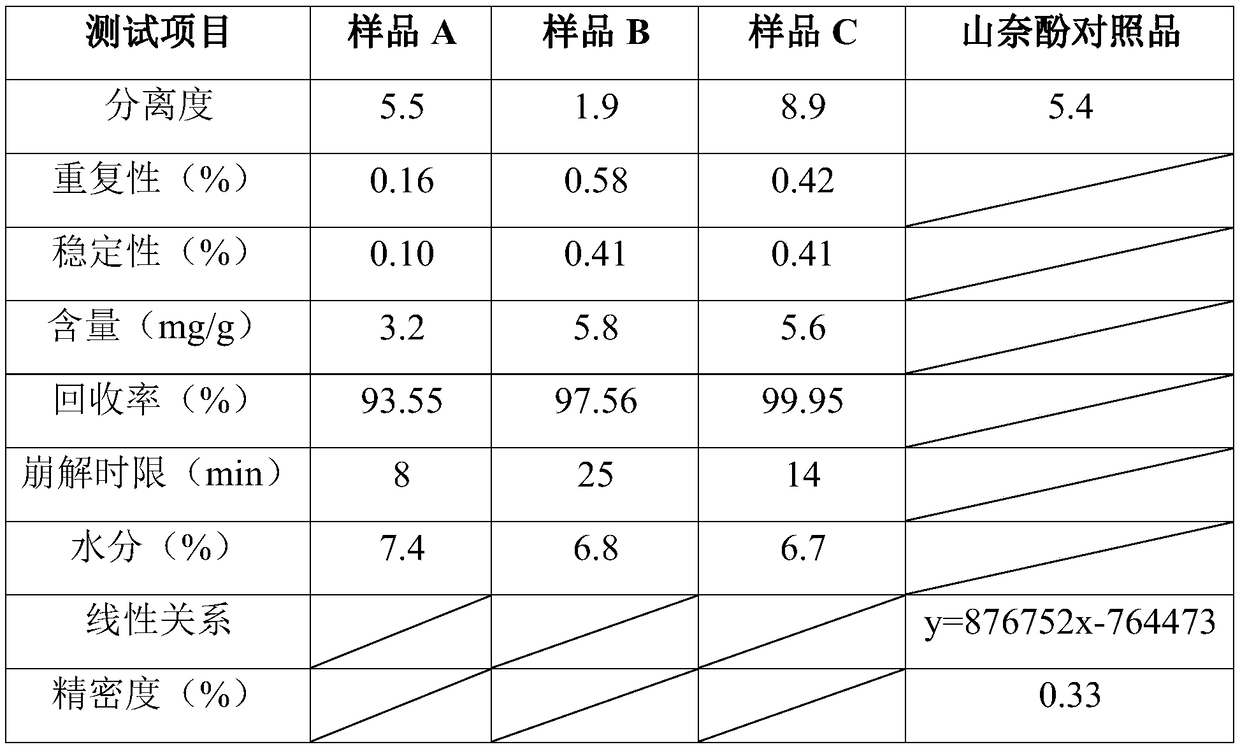
[0034] Chromatographic conditions: octadecylsilane bonded silica gel as filler; methanol-0.5% phosphoric acid solution (62:38) as mobile phase; detection wavelength: 368nm; column temperature: 30°C; flow rate: 1.0ml / min. The number of theoretical plates should not be less than 4000 based on the kaempferol peak.
[0035] Preparation of standard solution: Take an appropriate amount of kaempferol reference substance dried under reduced pressure by phosphorus pentoxide for 48 hours, weigh it accurately, add methanol to make a solution containing 40 μg of kaempferol per 1 ml, shake well, and obtain.
[0036] Need testing solution preparation: Grind A sample, B and C sample to powder respectively, take 1g, accurately weigh, add 10ml distilled water, heat to dissolve, let cool, slowly add ethanol 80ml, leave standstill for 30 minutes, filter, The residue was washed twice with a small amount of 85% ethanol, the filtrate and washing liqui...
Embodiment 2
[0039] Interference test:
[0040] According to the chromatographic conditions of Example 1, take kaempferol standard solution and 10 microliters each of A sample solution, B sample solution and C sample solution, inject a sample, record the chromatogram, the result is that the separation degree of kaempferol peak and other peaks is good , without interfering with the measurement results. The results of separation determination are shown in Table 1.
Embodiment 3
[0042] Linear relationship investigation:
[0043] According to the chromatographic conditions of Example 1, the kaempferol standard solution of 1, 5, 10, 15, and 20 microliters was respectively injected with an autosampler, and the chromatogram was recorded. The injection content (microgram) is the abscissa, and the chromatographic peak area value is the ordinate, and linear regression is performed to calculate the injection amount of the kaempferol standard solution and the peak area has a good linear relationship. The linear relationship results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com