Blue-light-excited fluorine manganite red-light-emitting material and preparation method and application thereof
A fluoromanganate, blue light excitation technology, applied in luminescent materials, chemical instruments and methods, electrical components and other directions, can solve the problems of complex experimental process, many steps, improve the defects of the material itself, etc., to achieve simple and easy preparation process, Mild conditions and improved photochromic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment K 2 MnF 6 The co-precipitation method of the red light material is prepared, and the specific preparation steps are as follows:
[0028] Weigh 10g KHF 2 Dissolve in 50ml of hydrofluoric acid solution with a mass fraction of 49%, add 1g KMnO 4 , stir until the solids are all dissolved, gradually add 30% hydrogen peroxide solution dropwise until the solution turns from purple to yellow, stop the drop immediately, collect the precipitated sample by filtration, wash with acetone for 3 times, and dry at 80°C 2h, get K 2 MnF 6 red light material.
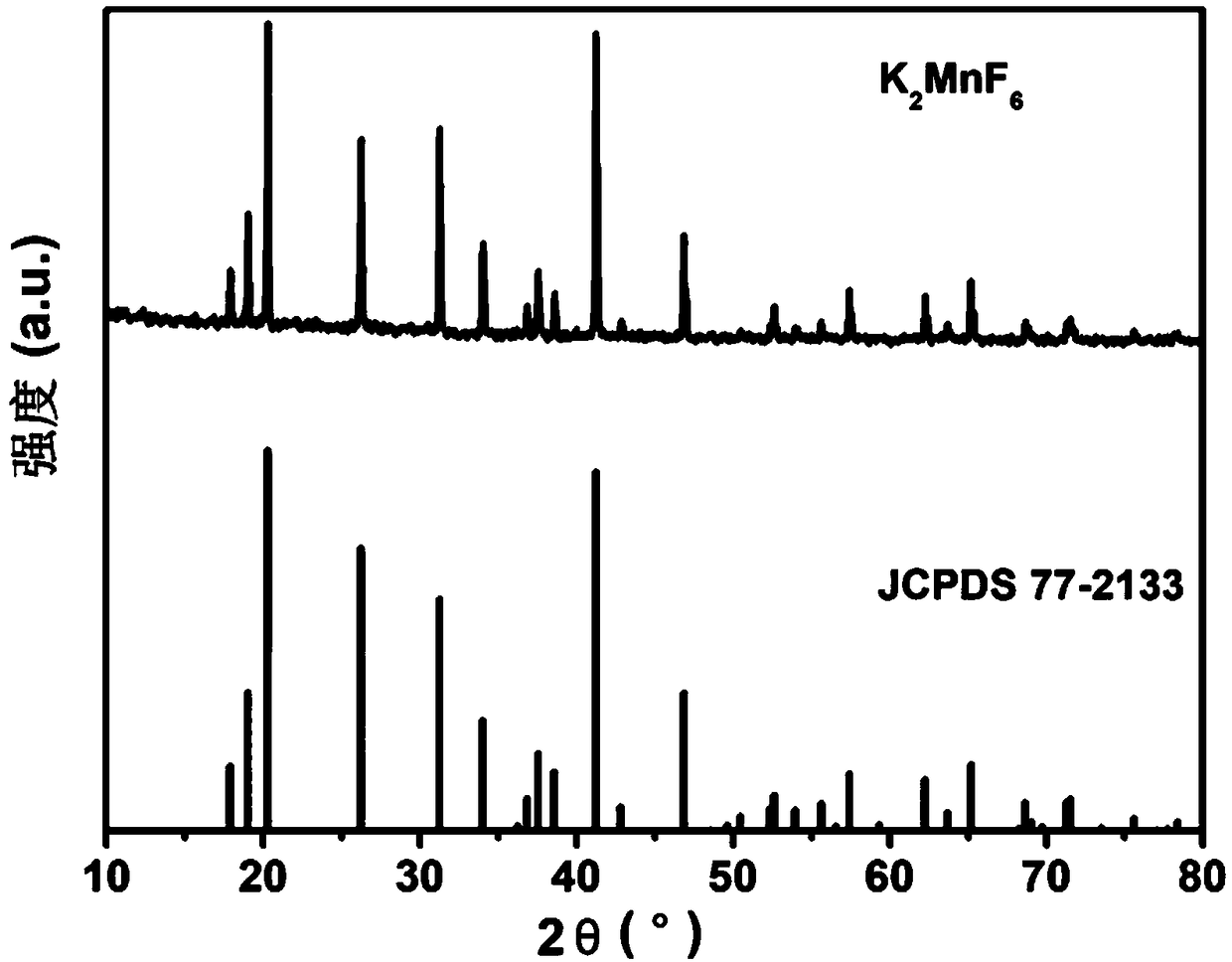
[0029] K obtained in this example 2 MnF 6 The XRD diffraction pattern of the red light material is as follows figure 1 shown by figure 1 It can be seen that the diffraction peak of the sample is consistent with that of the standard card JCPDS 77-1301 (K 2 MnF 6 ), there is no heterophase diffraction peak, indicating that the synthesized red light material sample is a pure phase.
[0030] K obtained in th...
Embodiment 2
[0032] In this example Cs 2 MnF 6 The red light material is prepared by the ion exchange method, and the specific preparation steps are as follows:
[0033] Weigh 2.46g K 2 MnF 6 Add to 10ml 40% hydrofluoric acid solution, add 6.06g CsF to react for 30 minutes, the obtained sample is filtered, washed and dried to obtain CsF 2 MnF 6 red light material.
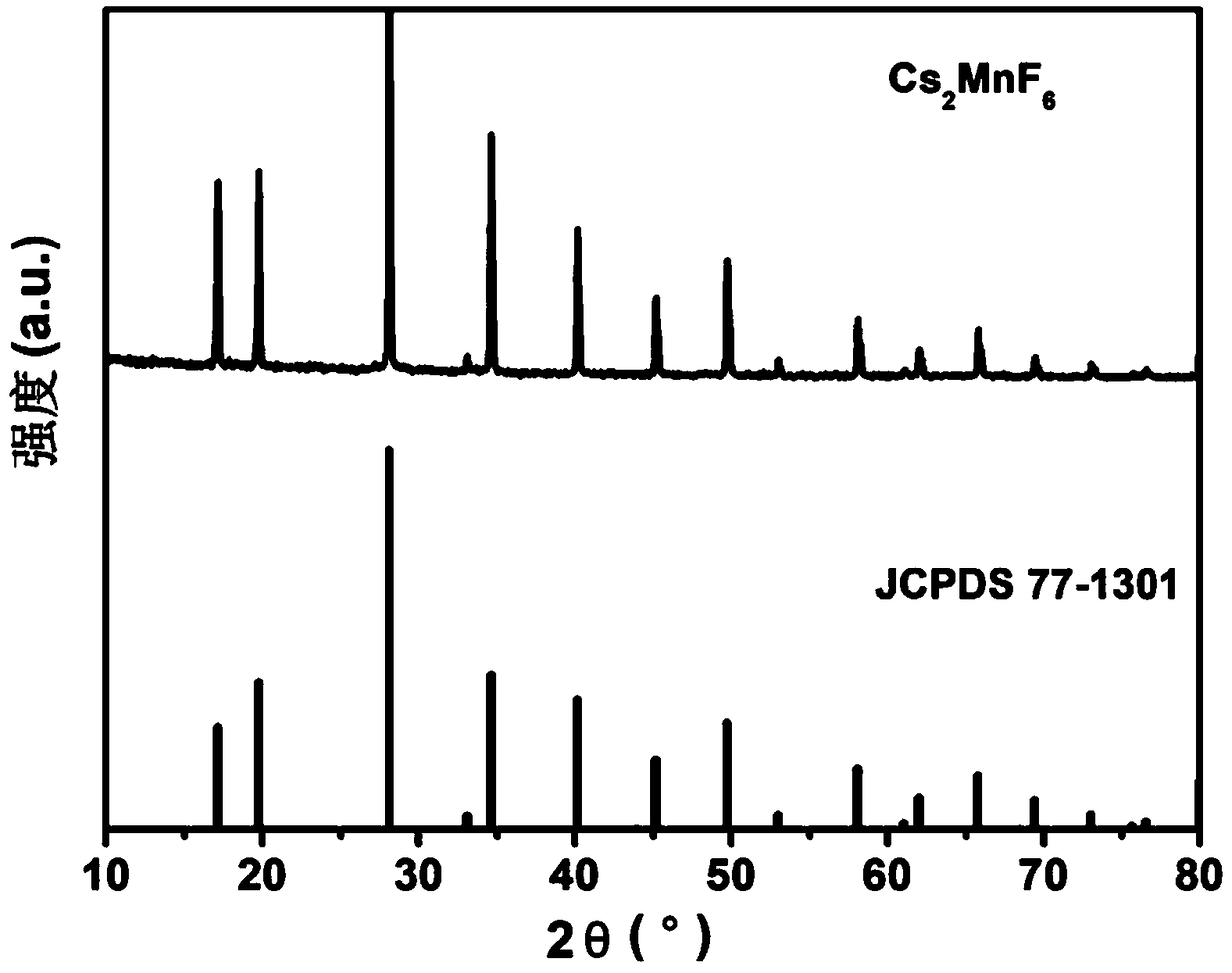
[0034] Cs obtained in this example 2 MnF 6 The XRD diffraction pattern of the red light material is as follows image 3 shown by image 3 It can be seen that the diffraction peak of the sample is consistent with the standard card JCPDS 77-1301 (Cs 2 MnF 6 ), there is no heterophase diffraction peak, indicating that the synthesized red light material sample is a pure phase.
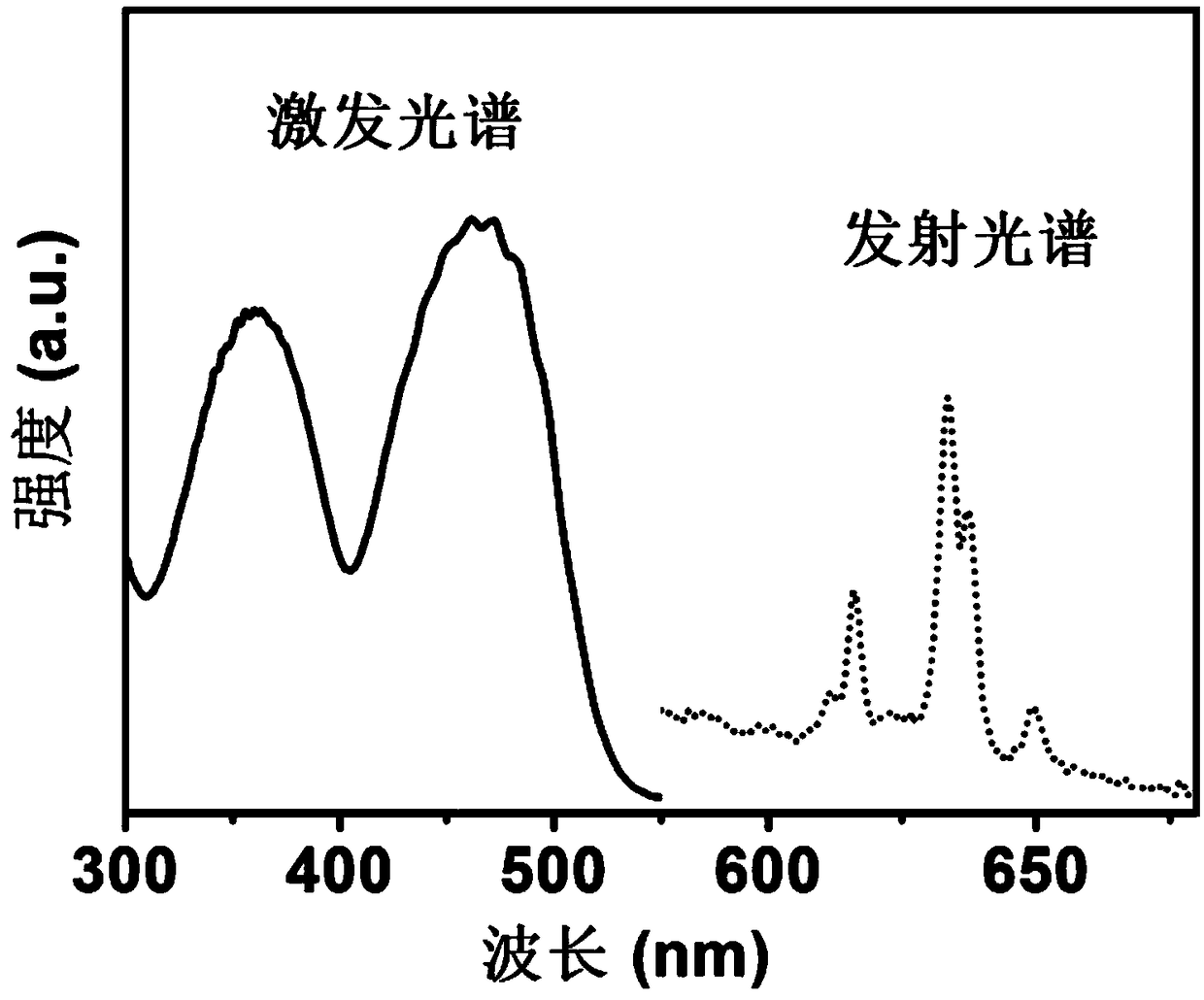
[0035] Cs obtained in this example 2 MnF 6 The room temperature excitation and emission spectra of red light materials are shown in Figure 4 shown by Figure 4 It can be seen that the sample has strong broadband excitation peaks in the ultra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com