Triptolide derivatives, and preparation method and applications thereof
A technology of triptolide and derivatives, applied in the pharmaceutical field, can solve the problems of large toxic and side effects hindering development and research, poor metabolic stability, increased poisoning, etc., so as to reduce physical, psychological and economic burdens, prolong half-life, and reduce clearance rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
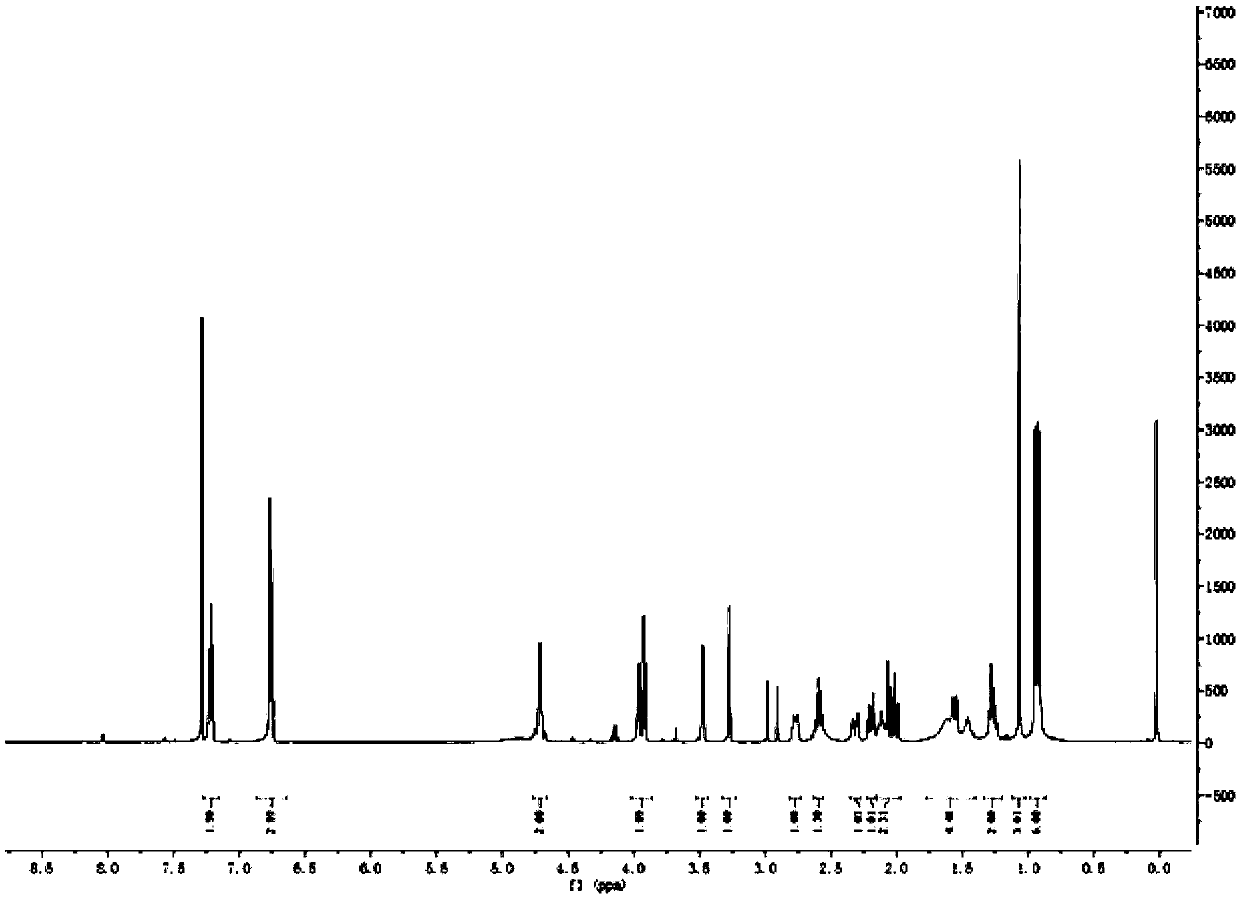
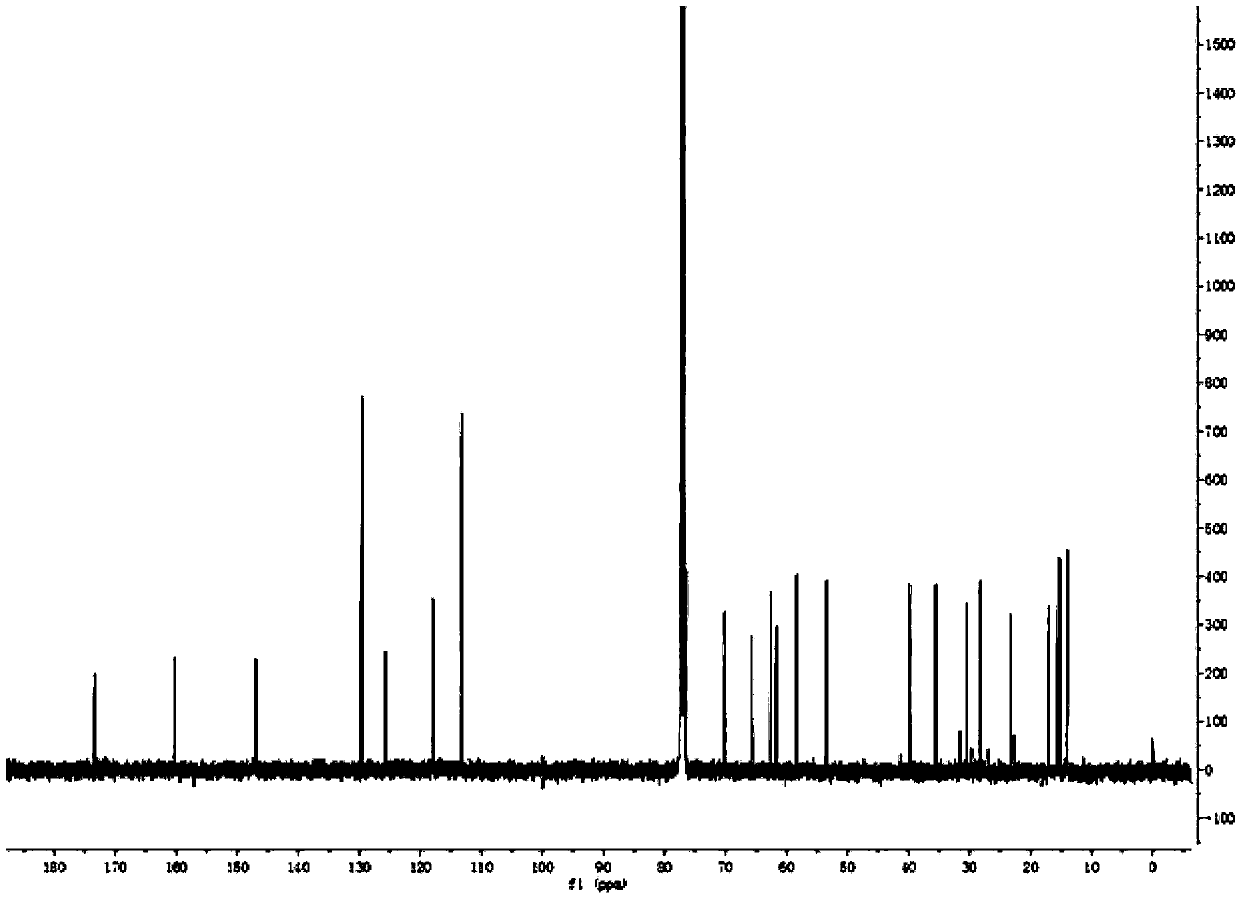
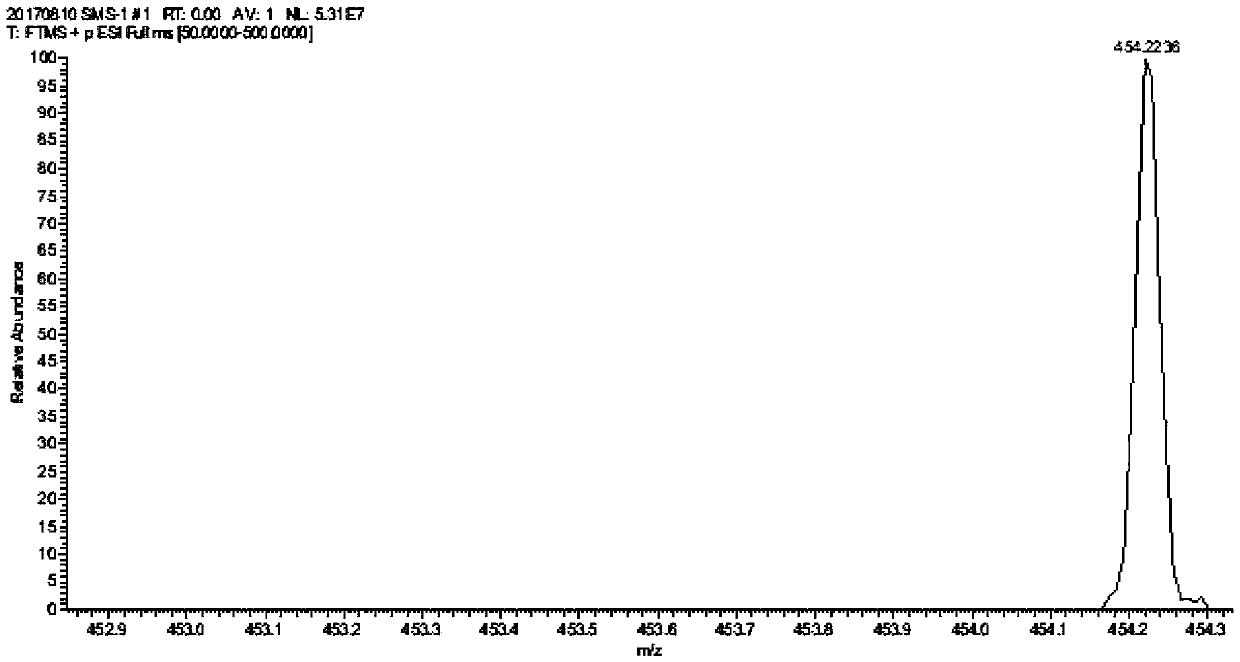
[0025] Preparation of Triptolide Derivatives
[0026] Add 50 mg of triptolide, 1.5 ml of morpholine, and 3.6 mg of ether magnesium bromide in a 25 ml reaction tube, and the reaction is carried out under solvent-free conditions. Stirring overnight at room temperature, the progress of the reaction was detected by thin-layer chromatography on a silica gel plate, the chromogen was kedde reagent, and triptolide and its derivatives were purple-red. After 48 hours of reaction, the reaction was complete, and ethyl acetate and deionized water were added for washing. The ethyl acetate phase was collected, dehydrated by adding anhydrous sodium sulfate, filtered, and concentrated to obtain a solid.
[0027] The solid was dissolved with a small amount of dichloromethane, separated and purified with a silica gel column, and eluted with petroleum ether: ethyl acetate = 5:1 as eluent. Use thin-layer chromatography to detect and collect the chromogenic fraction. Concentrate under reduced pr...
Embodiment 2
[0031] Triptolide derivatives inhibit cell viability curve and IC 50 Determination.
[0032] In this embodiment, the MTT method is used to measure the influence of triptolide derivatives on the proliferation of various cancer cells, and the specific operations are as follows:
[0033] (1) Digest the cells in the logarithmic growth phase with trypsin, add the medium, centrifuge and collect after the digestion is terminated, and make a cell suspension, and adjust the concentration to 5-10×10 by cell counting 4 individual / ml.
[0034] (2) Mix the cell suspension evenly, add 100 microliters to each well, the cell density in each well is 5000-10000 / well, and fill the edge wells with sterile PBS.
[0035] (3) Put the inoculated cells into the incubator and cultivate overnight, add drugs with different concentration gradients, and set three duplicate holes.
[0036] (4) 5% carbon dioxide, culture at 37 degrees Celsius for 72 hours, observe the cell viability with an inverted micro...
Embodiment 3
[0042] Determination of Half-life of Triptolide Derivatives
[0043] In this embodiment, the substrate elimination method under liver microsomes in vitro is adopted, and the specific operation is as follows:
[0044] (1) Add 1 μl of substrate to a 1.5ml EP tube, take a tris-HCl 15ml centrifuge tube, and take a certain amount of human liver microsomes (20mg / ml)
[0045] (2) Add alamethicin (2.5 μg / ml), vortex for 5 seconds, and ice-bath for 15 minutes to make holes for the microsomes.
[0046] (3) Magnesium chloride hexahydrate solution (100 mM), D-glucose-6-phosphate disodium salt (10 mM), and glucose-6-phosphate dehydrogenase (10 unit / ml) were added in sequence. Vortex for 10 sec to mix well.
[0047] (4) Take a certain amount of the mixed solution from the 15ml centrifuge tube and add it to each EP tube. Put it into a constant temperature mixer at 37°C, and pre-incubate for 10 minutes to balance.
[0048] (5) Add β-nicotinamide adenine dinucleotide phosphate (10 mM) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com