Iron complex on basis of flexible frameworks, method for preparing iron complex and application of iron complex to isoprene polymerization
A technology of iron complexes and flexible skeletons, applied in the direction of iron organic compounds, etc., can solve the problems of wide molecular weight distribution of polymers, high polymerization cost, and regulated polymerization by catalytic microstructure, and achieve the effect of simple synthesis method and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0035] This embodiment prepares the pyridinium imine iron complex shown in formula II:
[0036] 100mL dry reaction bottle, add 4A molecular sieve and bake for 30 minutes. Under argon atmosphere, dry dichloromethane (40 mL), 4-fluorobenzylamine (1.2 g, 9.3 mmol) and pyridine-2-carbaldehyde (1.0 g, 9.3 mmol) were added sequentially. The reaction was carried out overnight at room temperature, and the reaction of the aldehyde substrate was detected by TLC plate to complete the reaction. Filtration, spinning to dryness, and drying under vacuum gave a yellow liquid (1.9g, yield: 93%), the structural formula was
[0037] 25mL dry reaction tube, add 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl in the glove box 2 (100.0 mg, 0.8 mmol) and the above-prepared pyridine imine ligand (169.0 mg, 0.8 mmol) were stirred at room temperature for 15 h. After the reaction, dichloromethane was vacuum-dried, washed 3 times by adding 10 mL of dry n-hexane, and vacuum-dried ...
preparation example 2
[0041] This embodiment prepares the pyridinium imine iron complex shown in formula III:
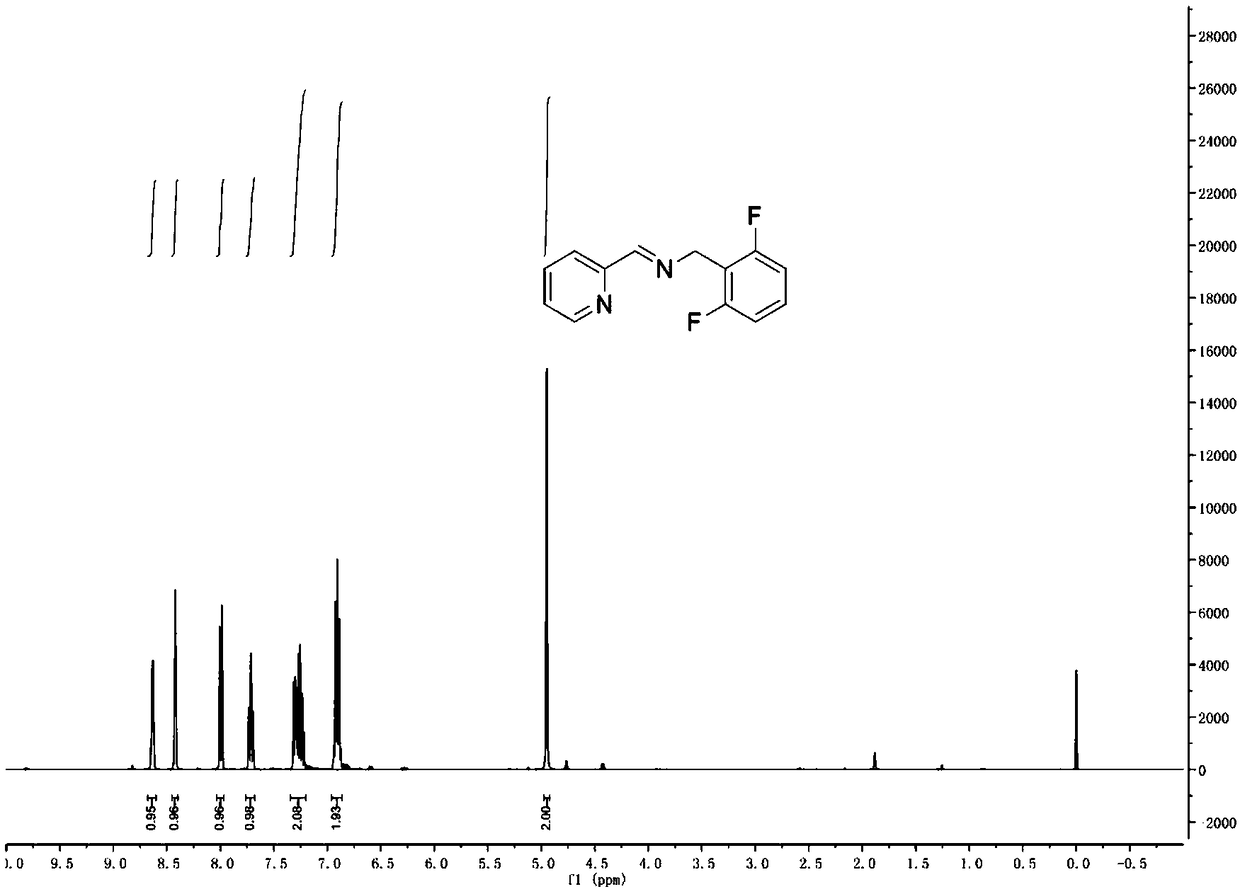
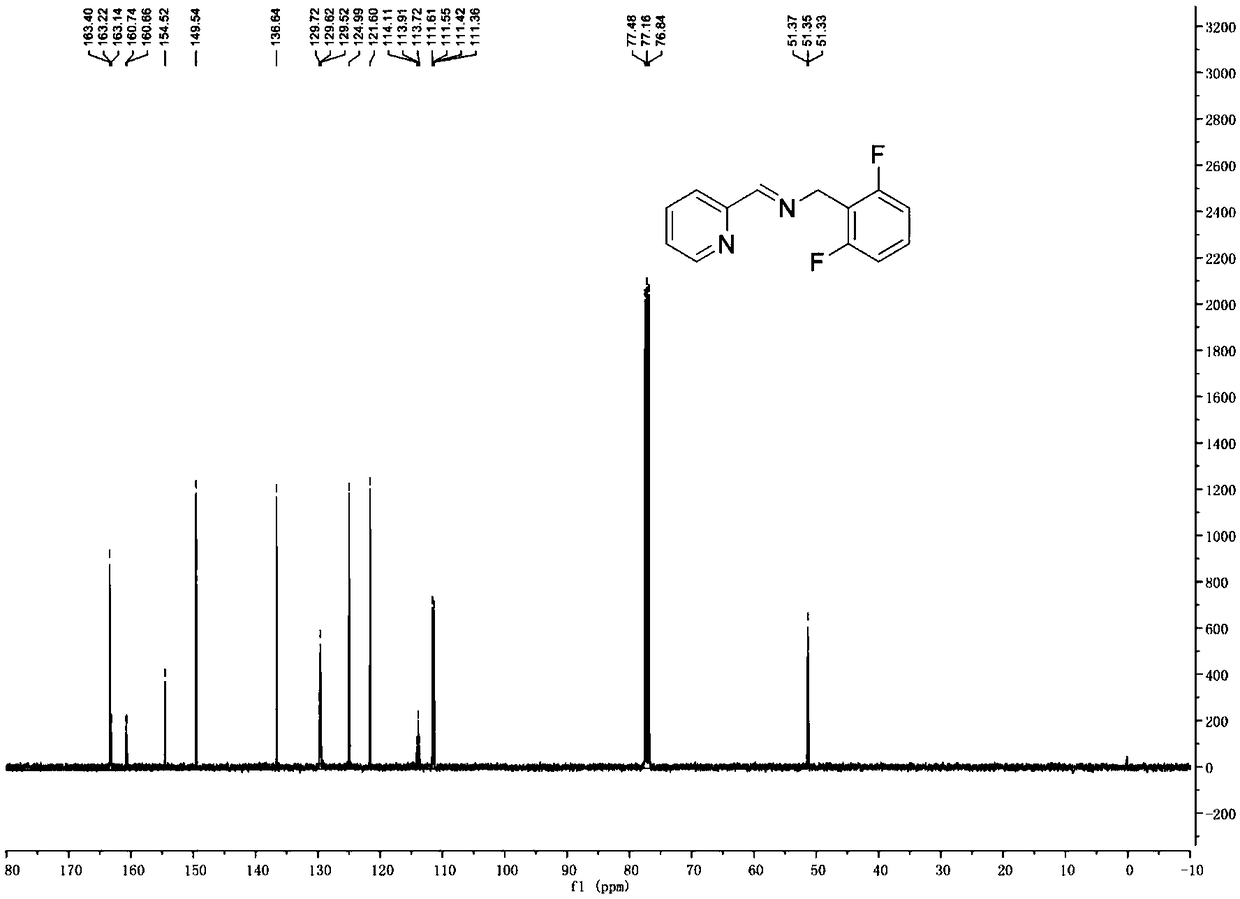
[0042]100mL dry reaction bottle, add 4A molecular sieve and bake for 30 minutes. Under argon atmosphere, dry dichloromethane (40 mL), 2,6-difluorobenzylamine (1.34 g, 9.34 mmol) and 2-pyridinecarbaldehyde (1.0 g, 9.34 mmol) were added sequentially. The reaction was carried out overnight at room temperature, and the reaction of the aldehyde substrate was detected by TLC plate to complete the reaction. Filtration, spinning to dryness, and drying under vacuum gave a yellow solid (1.7g, yield: 79%), the structural formula was
[0043] 25mL dry reaction tube, add 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl in the glove box 2 (100.0 mg, 0.8 mmol) and the above-prepared pyridine imine ligand (183.0 mg, 0.8 mmol) were stirred at room temperature for 15 h. After the reaction was completed, the dichloromethane was vacuum-dried, and 10 mL of dry n-hexane was added to was...
preparation example 3
[0047] The present embodiment prepares the pyridine imine iron complex shown in formula IV:
[0048] 100mL dry reaction bottle, add 4A molecular sieve and bake for 30 minutes. Under argon atmosphere, dry dichloromethane (60 mL), 4-trifluoromethylbenzylamine (2.5 g, 14.0 mmol) and pyridine-2-carbaldehyde (1.5 g, 14.0 mmol) were added sequentially. The reaction was carried out overnight at room temperature, and the reaction of the aldehyde substrate was detected by TLC plate to complete the reaction. Filtration, spinning to dryness, and drying under vacuum gave a yellow liquid (3.3g, yield: 90%), the structural formula was
[0049] 25mL dry reaction tube, add 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl in the glove box 2 (100.0 mg, 0.8 mmol) and the above-prepared pyridine imine ligand (208.5 mg, 0.8 mmol) were stirred at room temperature for 15 h. After the reaction, dichloromethane was vacuum-dried, washed with 10 mL of dry n-hexane for 3 times, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com