Compound ammonium glycyrrhetate S injection pharmaceutical composition as well as preparation method and application thereof
A technology of monoammonium glycyrrhizinate and monoammonium glycyrrhizinate, which is applied in the field of medicine, can solve the problems of side effects, poor stability, single effect and curative effect, etc., and achieve the effect of improving stability, good stability and remarkable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
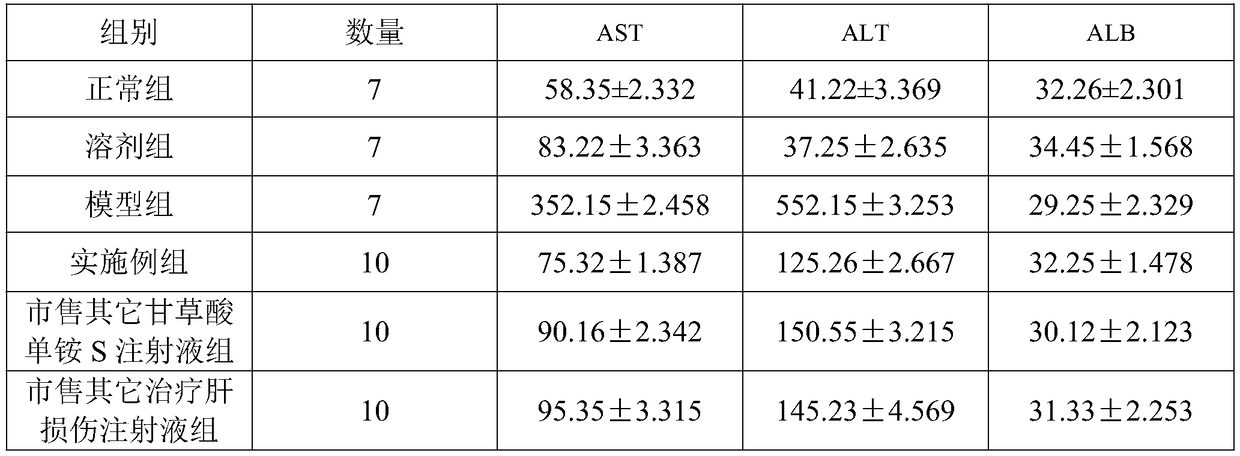
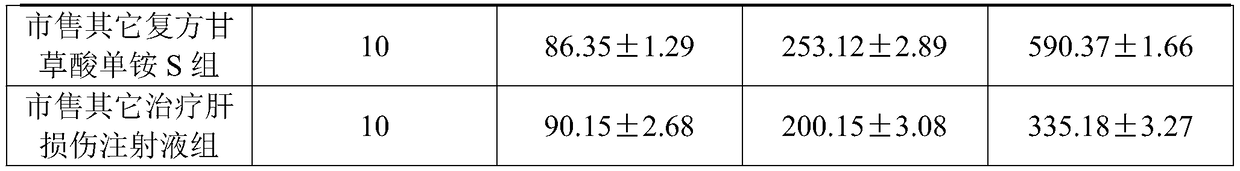
[0029] A compound monoammonium glycyrrhizinate S injection pharmaceutical composition, comprising the following raw materials in parts by weight:
[0030] 400g monoammonium glycyrrhizinate S, 320g cysteine hydrochloride, 4.0kg glycine, 400g anhydrous sodium sulfite, 45g disodium edetate, 1.0kg sodium chloride, 30g sodium citrate, 21g citric acid, 280ml capillary Extract, Radix Bupleurum extract, Curcuma turmeric extract, Radix Radix Radix and Phellodendron Phellodendron extract by reflux extraction, and finally dilute to 200L with water for injection.
[0031] The preparation method of this compound monoammonium glycyrrhizinate S injection pharmaceutical composition comprises the following steps:
[0032] S1. Weigh glycyrrhizinate monoammonium salt S, cysteine hydrochloride, glycine, sodium citrate, anhydrous sodium sulfite, disodium edetate, citric acid, sodium chloride and Chinese medicinal materials; Weighing and dissolving charcoal in the charcoal storage and weighing...
Embodiment 2
[0039] A compound monoammonium glycyrrhizinate S injection pharmaceutical composition, comprising the following raw materials in parts by weight:
[0040] 430g monoammonium glycyrrhizinate S, 300g cysteine hydrochloride, 4.2kg glycine, 450g anhydrous sodium sulfite, 40g disodium edetate, 1.2kg sodium chloride, 40g sodium citrate, 25g citric acid, 300ml capillary extract, Radix Bupleuri extract, Curcuma extract, Radix Radix Radix and Phellodendron Phellodendron extract by reflux extraction, and finally dilute to 210L with water for injection.
[0041] A preparation method of compound monoammonium glycyrrhizinate S injection pharmaceutical composition, is characterized in that, comprises the following steps:
[0042] S1. Weigh glycyrrhizinate monoammonium salt S, cysteine hydrochloride, glycine, sodium citrate, anhydrous sodium sulfite, disodium edetate, citric acid, sodium chloride and Chinese medicinal materials; Weighing and dissolving charcoal in the charcoal storage an...
Embodiment 3
[0049] A compound monoammonium glycyrrhizinate S injection pharmaceutical composition, comprising the following raw materials in parts by weight:
[0050] 500g monoammonium glycyrrhizinate S, 400g cysteine hydrochloride, 4.5kg glycine, 500g anhydrous sodium sulfite, 50g disodium edetate, 1.5kg sodium chloride, 60g sodium citrate, 50g citric acid, 350ml capillary Extract, Radix Bupleurum Extract, Curcuma Radix Extract, Radix Isatidis Extract and Phellodendron Phellodendron Extract by reflux extraction, and finally dilute to 280L with water for injection.
[0051] A preparation method of compound monoammonium glycyrrhizinate S injection pharmaceutical composition, is characterized in that, comprises the following steps:
[0052] S1. Weigh glycyrrhizinate monoammonium salt S, cysteine hydrochloride, glycine, sodium citrate, anhydrous sodium sulfite, disodium edetate, citric acid, sodium chloride and Chinese medicinal materials; Weighing and dissolving charcoal in the charcoa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Filter pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com