Nickel-based composite catalyst for methane reforming with carbon dioxide to produce synthesis gas under pressurization
A composite catalyst and carbon dioxide technology, applied in metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrogen/synthesis gas production, etc., can solve the problem that the reforming methane reaction cannot continue and is difficult to achieve commercialization Application, rapid catalyst deactivation and other problems, to achieve the effect of inhibiting carbon deposition, increasing dispersion and surface active metal content, and improving catalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
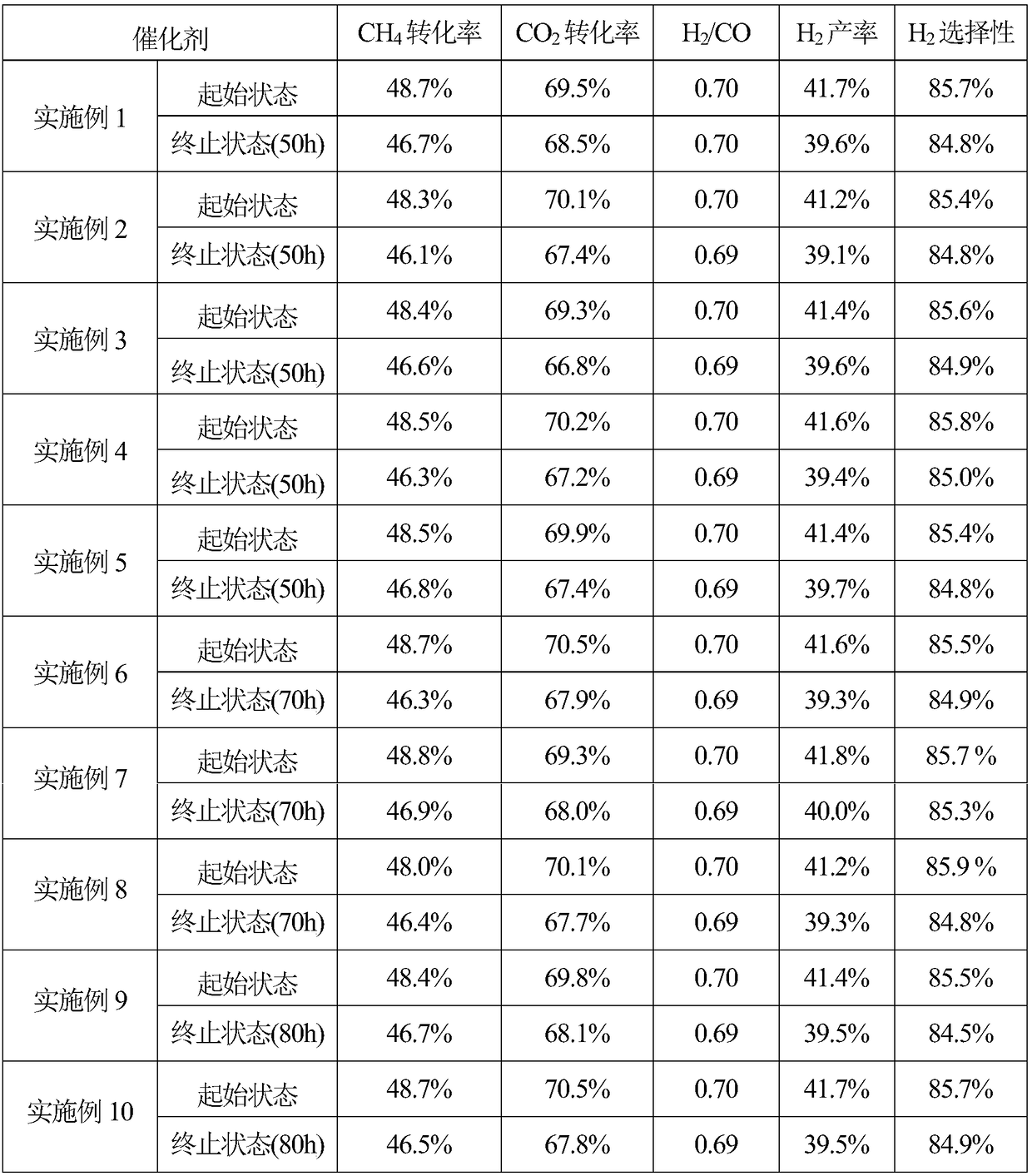
Examples
Embodiment 1
[0022] According to CeO 2 -La 2 o 3 CeO in composite oxide 2 with La 2 o 3 The mass ratio is 3:2, 0.986g (0.17mmol) of P123 with a number average molecular weight of 5800 and 2.5471g (5.867mmol) of cerium nitrate hexahydrate, 1.7894g (4.132mmol) of lanthanum nitrate hexahydrate are added in 20mL of absolute ethanol , stirred at room temperature for 5 hours to completely dissolve the solid, and the resulting mixture was transferred to a Petri dish, covered with a perforated PE film, and then transferred to a blast oven at 40 °C for solvent evaporation for 48 hours, and then evaporated at 100 °C for 24 hours. Finally, the obtained xerogel was heated to 450°C at a heating rate of 1°C / min under the air atmosphere flowing in a tube furnace, roasted at a constant temperature for 4 hours, cooled naturally to room temperature, and ground into powder to obtain the desired CeO2 -La 2 o 3 composite oxides.
[0023] 10%Ni-3%CeO according to catalyst composition 2 -2% La 2 o 3 -S...
Embodiment 2
[0025] According to CeO 2 -Sm 2 o 3 CeO in composite oxide 2 with Sm 2 o 3 The mass ratio is 4:3, 0.986g (0.17mmol) of P123 with a number average molecular weight of 5800 and 2.4946g (5.746mmol) of cerium nitrate hexahydrate, 1.8907g (4.254mmol) of samarium nitrate hexahydrate are added in 20mL of absolute ethanol , stirred at room temperature for 5 hours to completely dissolve the solid, and the resulting mixture was transferred to a Petri dish, covered with a perforated PE film, and then transferred to a blast oven at 40 °C for solvent evaporation for 48 hours, and then evaporated at 100 °C for 24 hours. Finally, the obtained xerogel was heated to 450°C at a heating rate of 1°C / min under the air atmosphere flowing in a tube furnace, roasted at a constant temperature for 4 hours, cooled naturally to room temperature, and ground into powder to obtain CeO 2 -Sm 2 o 3 composite oxides.
[0026] According to the catalyst composition is 10%Ni-4%CeO 2 -3% Sm 2 o 3 -SiO ...
Embodiment 3
[0028] According to CeO 2 -Pr 6 o 11 CeO in composite oxide 2 with Pr 6 o 11 The mass ratio of 0.986g (0.17mmol) of P123 with a number average molecular weight of 5800 and 2.8836g (6.642mmol) of cerium nitrate hexahydrate, 1.4606g (3.3576mmol) of praseodymium nitrate hexahydrate were added in 20mL of absolute ethanol , stirred at room temperature for 5 hours to completely dissolve the solid, and the resulting mixture was transferred to a Petri dish, covered with a perforated PE film, and then transferred to a blast oven at 40 °C for solvent evaporation for 48 hours, and then evaporated at 100 °C for 24 hours. Finally, the obtained xerogel was heated to 450°C at a heating rate of 1°C / min under the air atmosphere flowing in a tube furnace, roasted at a constant temperature for 4 hours, cooled naturally to room temperature, and ground into powder to obtain CeO 2 -Pr 6 o 11 composite oxides.
[0029] According to the catalyst composition is 10%Ni-4%CeO 2 -2%Pr 6 o 11 -S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com