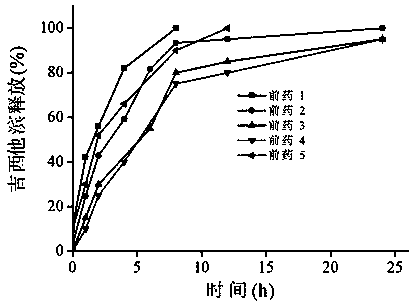
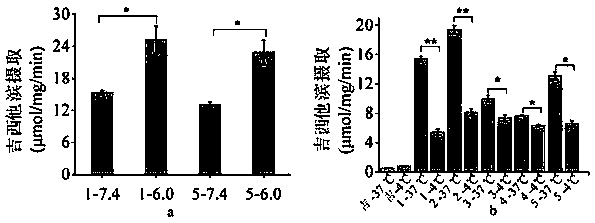
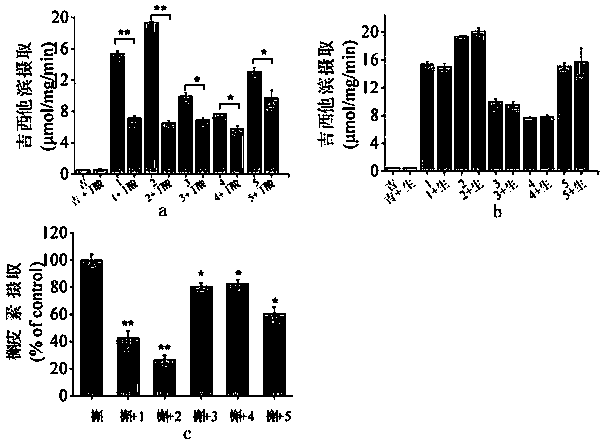
Prodrugs designed on basis of intestinal MCT1 (monocarboxylate transporter 1) carrier protein and preparation method of prodrugs
A technology of MCT1 and prodrug, applied in the field of medicine, can solve the problems of incomplete release of the parent drug, poor permeability of antitumor drugs, low oral bioavailability, etc., so as to improve oral bioavailability, improve drug efficacy, and increase patients The effect of compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0055] Example 1 gemcitabine-succinate monoester prodrug preparation (prodrug 1)
[0056] Dissolve 0.35g (3mmol) of succinic acid in 15ml of anhydrous dioxane, add 0.25g (2.5mmol) of triethylamine, dropwise add 0.28g (2.4mmol) of thionyl chloride, and vacuum the nitrogen after dropping Protected, refluxed at 110°C for 4h, concentrated the reaction solution under reduced pressure to obtain a light yellow oil, which was redissolved in 10ml DMF. 0.78 g (3.2 mmol) of gemcitabine was added, and 0.19 g (1.5 mmol) of triethylamine was added dropwise at room temperature. After the dropping was completed, the mixture was vacuumed under nitrogen protection and reacted overnight, and the reaction was complete as monitored by TLC. The reaction solution was concentrated under reduced pressure to obtain a reddish-brown oil. Column chromatography gave a white solid with a yield of 75%. MS(ESI): m / z=364[M+H + ]. The purity determined by HPLC was 97.5%. 1 H NMR (400MHz,D 2 O) δ7.73(d, J=...
example 4
[0061] Example 4 gemcitabine-suberate monoester prodrug preparation (prodrug 4)
[0062] Dissolve 0.61g (3mmol) of sebacic acid in 15ml of anhydrous dioxane, add 0.25g (2.5mmol) of triethylamine, then add 0.28g (2.4mmol) of thionyl chloride dropwise, and vacuum nitrogen after dropping Protected, refluxed at 110°C for 4h, concentrated the reaction solution under reduced pressure to obtain a light yellow oil, which was redissolved in 10ml DMF. 0.78 g (3.2 mmol) of gemcitabine was added, and 0.19 g (1.5 mmol) of triethylamine was added dropwise at room temperature. After the dropping was completed, the mixture was vacuumed under nitrogen protection and reacted overnight, and the reaction was complete as monitored by TLC. The reaction solution was concentrated under reduced pressure to obtain a reddish-brown oil. Column chromatography gave a white solid with a yield of 53%. MS(ESI): m / z=448[M+H + ]. The purity determined by HPLC was 97.6%. 1H NMR (400MHz, DMSO) δ11.81(s, 1H), ...
example 5
[0063] Example 5 gemcitabine-succinate prodrug preparation (prodrug 5)
[0064] Dissolve 0.35g (3mmol) of succinic acid in 15ml of anhydrous dioxane, add 0.25g (2.5mmol) of triethylamine, dropwise add 0.28g (2.4mmol) of thionyl chloride, and vacuum the nitrogen after dropping Protected, refluxed at 110°C for 4h, concentrated the reaction solution under reduced pressure to obtain a light yellow oil, which was redissolved in 10ml DMF. 0.39 g (1.6 mmol) of gemcitabine was added, and 0.09 g (0.9 mmol) of triethylamine was added dropwise at room temperature. After the dropping, vacuum was applied under nitrogen protection, and the reaction was carried out overnight, and the reaction was monitored by TLC to complete. The reaction solution was concentrated under reduced pressure to obtain a reddish-brown oil. Column chromatography gave a white solid with a yield of 55%. MS(ESI): m / z=464[M+H + ]. The purity determined by HPLC was 98.3%. 1 H NMR (400MHz, MeOD) δ8.01(s, 1H), 7.55(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com