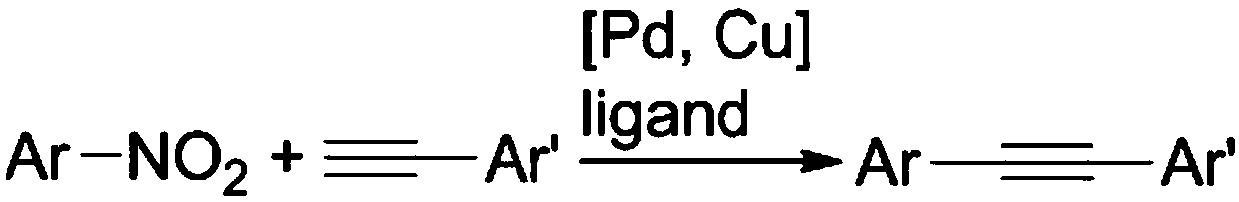Method for preparing aromatic alkynes through cross-coupling of nitroaromatic hydrocarbons and aryl-terminated alkynes under catalysis of transition metal
A transition metal catalyzed, nitroaromatic hydrocarbon technology, applied in the direction of condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, halogenated hydrocarbon preparation, organic chemical methods, etc. Achieving a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] At room temperature, add 0.5mmol nitrobenzene, 0.6mmol phenylacetylene, 0.05mmol Pd(acac) into a 50mL two-necked flask 2 , 0.05mmol CuI, 0.1mmol BrettPhos, 2.0mmol i-Pr 2 NH and 10 mL of toluene, and then the flask was placed in an oil bath reactor with magnetic stirring, and the reaction was carried out at 100 ° C for 18 h. After the reaction was completed, 10 mL of n-hexane was added for extraction and separation, and the product diphenylacetylene was obtained by column chromatography with a yield of 93%.
[0028] Diphenylacetylene is white powder
[0029] 1 H NMR (500MHz, CDCl 3 ):δ7.32-7.37(m,6H),7.52-7.56(m,4H).
Embodiment 2
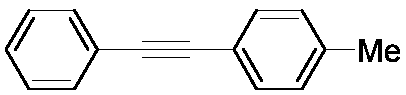
[0031] The structural formula of the target product is as follows:
[0032]
[0033]The steps are the same as in Example 1, using nitrobenzene and 4-methylphenylacetylene as raw materials.
[0034] The target product is a white solid with a yield of 92%.
[0035] 1 H NMR (500MHz, CDCl 3 ):δ2.36(s,3H),7.16(d,J=7.9Hz,2H),7.30-7.35(m,3H),7.43(d,J=7.9Hz,2H),7.52-7.54(m, 2H); 13 C NMR (125MHz, CDCl 3 ): δ21.49, 88.67, 89.52, 120.10, 123.39, 128.05, 128.29, 129.08, 131.45, 131.50, 138.35.
Embodiment 3
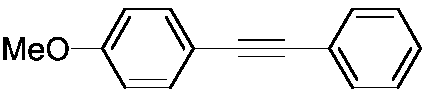
[0037] The structural formula of the target product is as follows:
[0038]
[0039] The steps are the same as in Example 1, using p-nitroanisole and phenylacetylene as raw materials.
[0040] The target product is a white solid with a yield of 88%.
[0041] 1 H NMR (500MHz, CDCl 3 ): δ3.83(s,3H),6.87(d,J=8.8Hz,2H),7.29-7.36(m,3H),7.46-7.48(m,2H),7.50-7.52(m,2H); 13 C NMR (125MHz, CDCl 3 ): δ55.26, 88.04, 89.35, 113.96, 115.34, 123.56, 127.92, 128.25, 131.42, 133.03, 159.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com