High performance liquid chromatography method for determining nicergoline related substances
A high-performance liquid chromatography and nicergoline technology, applied in the field of drug analysis, can solve the problems of unstable retention time of impurities, difficulty in obtaining impurity reference substances, and high price of impurity reference substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The verification of embodiment 1 chromatographic conditions
[0075] Chromatographic column: Filler with a mixture of strong cation exchange resin and reverse phase C18 in a volume ratio of 1:4;
[0076] Mobile phase: Acetonitrile is used as mobile phase A, and phosphate buffer (60 mmol / L potassium dihydrogen phosphate solution containing 0.5% triethylamine, adjusted to pH 2.1 with phosphoric acid) is used as mobile phase B. The ratio of mobile phase A and mobile phase B is 30:70 for isocratic elution;
[0077] Detection wavelength: 288nm;
[0078] Column temperature: 30°C;
[0079] Preparation of impurity test solution: take one EP system applicability reference substance, add 1ml of acetonitrile to dissolve it, and get it;
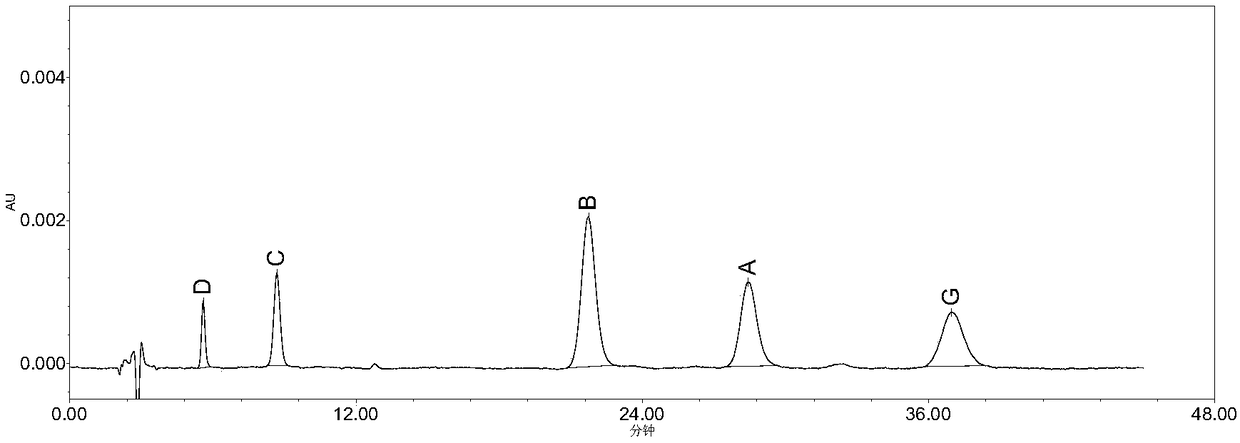
[0080] The preparation of mixed impurity reference substance solution: get impurity D 9.42mg, impurity A (lonyergoline) 10.68mg, impurity G (1-desmethoxynicergoline) 10.25mg, impurity B (1-norniergoline) ) 10.30mg, impurity C 10.30mg, put in 1...
Embodiment 2
[0085] The thermal stability investigation of embodiment 2 Nicergoline
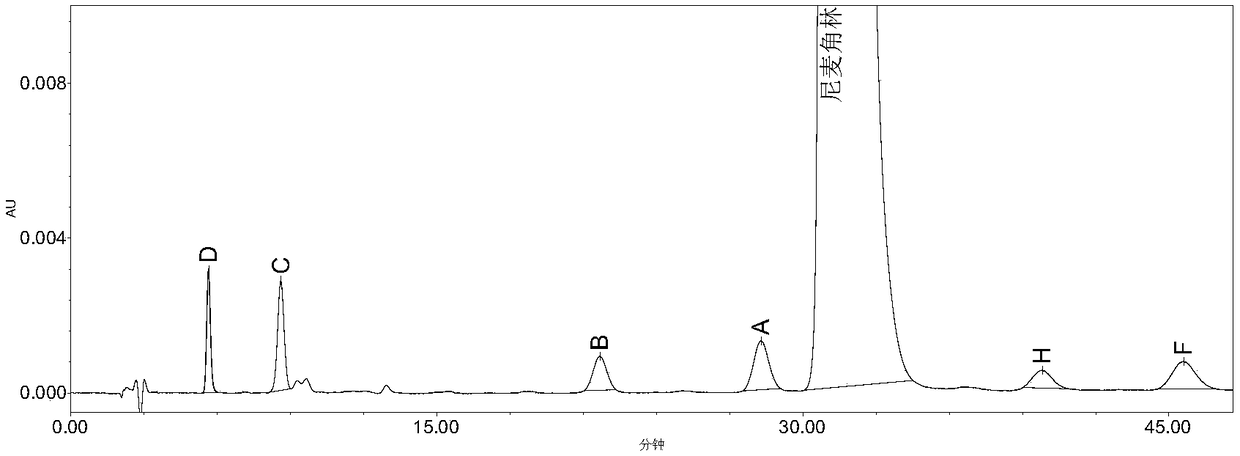
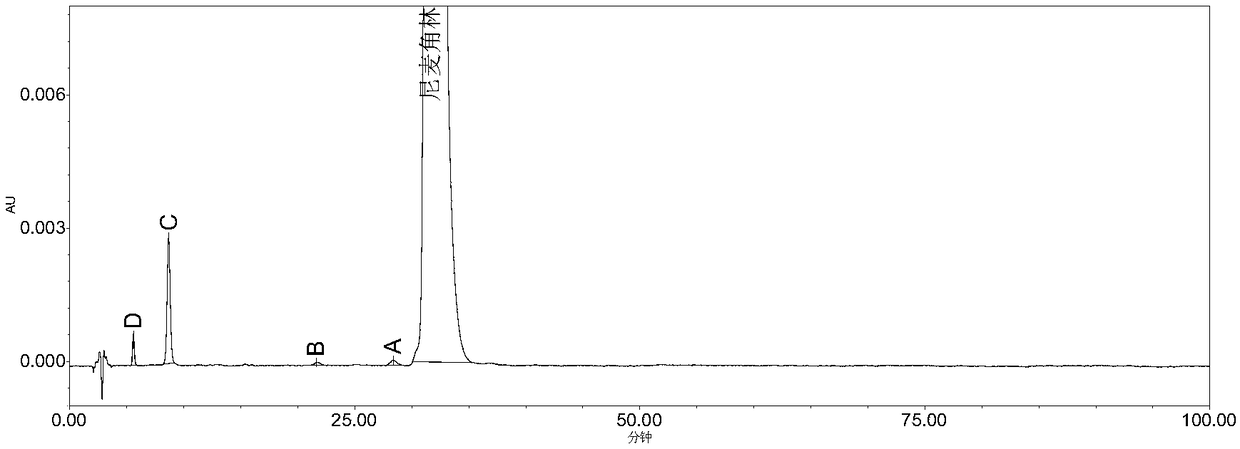
[0086] Accurately weigh 0.1028g of the Nicergoline reference substance, put it in a 100ml measuring bottle, add acetonitrile-water (8:2) to dissolve and dilute to the mark, shake well, and use it as the test solution. Take the test solution and heat it in a water bath at 60°C to destroy it, and accurately measure 20 μl of the test solution heated for 0 min, 10 min, 30 min, and 50 min respectively and inject it into the liquid chromatograph, record the chromatogram according to the chromatographic conditions in Example 1, see Figure 4~7 , the results showed that Nicergoline was thermally degraded, and impurity D and impurity C gradually increased with heating time, see Table 1 for details.
[0087] Table 1
[0088]
Embodiment 3
[0089] Embodiment 3 linear range, detection limit and quantitative limit
[0090] Take impurity D 9.42mg, impurity A (lonyergoline) 10.68mg, impurity G (1-desmethoxynicergoline) 10.25mg, impurity B (1-norniergoline) 10.30mg, impurity C 10.30, Nicergoline reference substance 10.12 mg was put in a 10ml measuring bottle respectively, dissolved with acetonitrile and diluted to the mark as each reference substance stock solution. Accurately measure an appropriate amount of each reference substance stock solution, dilute with acetonitrile to make a series of solutions, and accurately measure 10 μL respectively according to the chromatographic conditions of Example 1 and inject it into a liquid chromatograph, and record the chromatogram. In concentration (μg·ml -1 ) is x, and taking the peak area as y, calculate the linear regression equation and the correction factor (the ratio of the slope of Nicergoline to the slope of each component) of each component respectively, the results a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com