Preparation method of au-ir nanochain electrocatalyst for water splitting and oxygen production
An electrocatalyst and nanochain technology, applied in nanotechnology, electrodes, electrolysis components, etc., to achieve the effect of industrialized hydrogen production, high yield and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
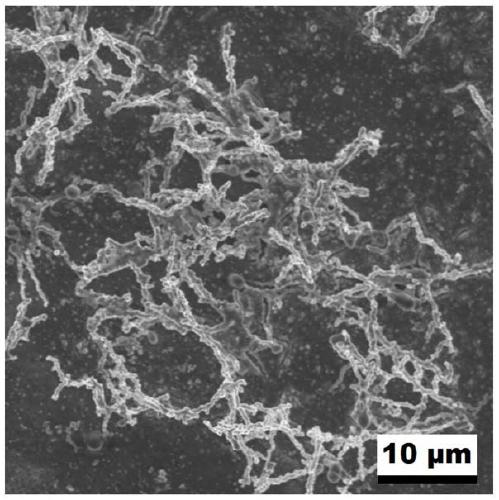
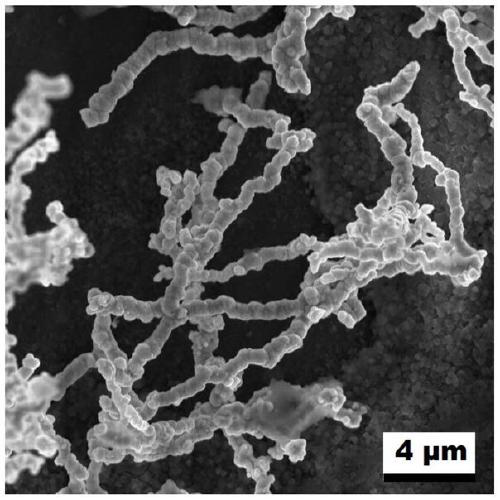
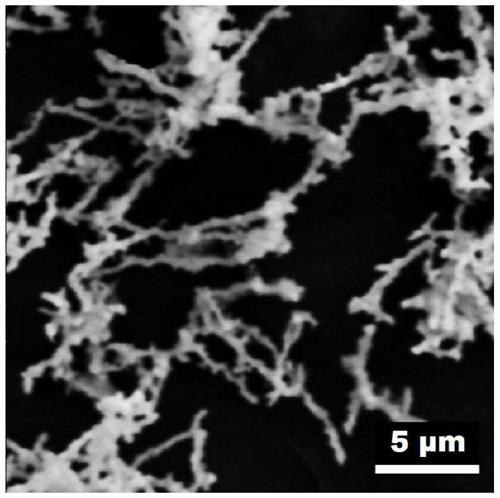
[0024] Stir 2mL 0.5mol / L chloroauric acid aqueous solution and 2mL 0.5mol / L potassium bromide aqueous solution, then add 2mL 0.5mol / L iridium chloride aqueous solution and stir evenly, and adjust the pH of the mixture to 3 with hydrochloric acid, and finally add 1mL (0.027mmol) of formaldehyde was stirred evenly, and left to react at 180°C for 10 hours under airtight conditions, cooled to room temperature, centrifuged, washed with distilled water for 3 to 5 times, and dried in a vacuum oven at 60°C to obtain Au-Ir nano chain electrocatalyst. Depend on Figure 1~3 It can be seen that the prepared sample is a one-dimensional nano-chain structure with uniform size and rough surface. Depend on Figure 4 It can be seen that there are five diffraction peaks at 38.187, 44.385, 64.576, 77.567 and 81.722°, which are consistent with the standard card of Au(fcc) (PDF#65-2870); five are at 40.662, 47.313, 69.142, 83.445 and 88.066° The diffraction peaks are consistent with the standard...
Embodiment 2
[0026] Stir 2mL 0.5mol / L chloroauric acid aqueous solution and 1mL 0.5mol / L potassium bromide aqueous solution, then add 2mL 0.5mol / L iridium trichloride aqueous solution and stir evenly, and adjust the pH of the mixed solution to 5 with hydrochloric acid, and finally add 0.85mL (0.023mmol) of formaldehyde was stirred evenly, and left to react at 160°C for 8 hours under airtight conditions, cooled to room temperature, centrifuged, washed with distilled water for 3 to 5 times, and dried in a vacuum oven at 60°C to obtain Au-Ir nanochain electrocatalysts (see Figure 9 ).
Embodiment 3
[0028] 2mL 0.5mol / L chloroauric acid aqueous solution and 1mL 0.5mol / L potassium bromide aqueous solution were stirred evenly, then added 1.5mL 0.5mol / L iridium chloride aqueous solution and stirred evenly, and the pH of the mixed solution was adjusted to 4 with hydrochloric acid, and finally Add 1.2mL (0.033mmol) formaldehyde and stir evenly, let stand at 200°C for 12 hours under airtight conditions, cool to room temperature, centrifuge, wash with distilled water for 3 to 5 times, and dry in a vacuum oven at 60°C to obtain Au- Ir nanochain electrocatalysts (see Figure 10 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com