Mutant of PET (polyethylene terephthalate) degrading enzyme and application of PET degrading enzyme
A mutant and degrading enzyme technology, applied in the field of genetic engineering, can solve the problem of low activity of IsPETase, achieve the effect of improved hydrolysis activity and efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The wild-type IsPETase was cloned into a commercially available pET28a+ vector by restriction enzyme ligation. Then, IsPETase was subjected to site-directed mutation using a commercially available site-directed mutagenesis reagent, and transformed into E. coli BL21 (DE3) to obtain an E. coli strain of PET degrading enzyme.
Embodiment 2
[0044]In order to obtain wild-type IsPETase and its mutant proteins, the wild-type IsPETase and mutant Escherichia coli strains obtained in Example 1 were cultured at 37° C. in the medium of LB plus ampicillin, and grown to an OD600 of about 0.6-0.8. Afterwards, the culture was cooled to 16 °C and induced with IPTG at a final concentration of 0.1 mM at 16 °C and 160 rpm for 20 h. Cells were harvested by centrifugation (25 min, 10°C, 4,000 rpm). Resuspend the cell pellet from 200 mL of cell culture in 10 mL of Ni-NTA lysis buffer (50 mM NaH 2 PO 4 , 300mM NaCl, 10mM imidazole, pH8). Cells were disrupted by sonication on ice and lysates were centrifuged (30 min, 10°C, 4,000 rpm). The supernatant was applied to a His-Accept nickel column (Beyotime). After washing unbound protein with 10 mL of lysis buffer, the bound protein was eluted with elution buffer (50 mM Tris-HCl pH 7.5, 300 mM NaCl, 200 mM imidazole), buffer exchanged to 50 mM Na 2 HPO 4 - HCl, pH 7.0 and 100 mM NaC...
Embodiment 3
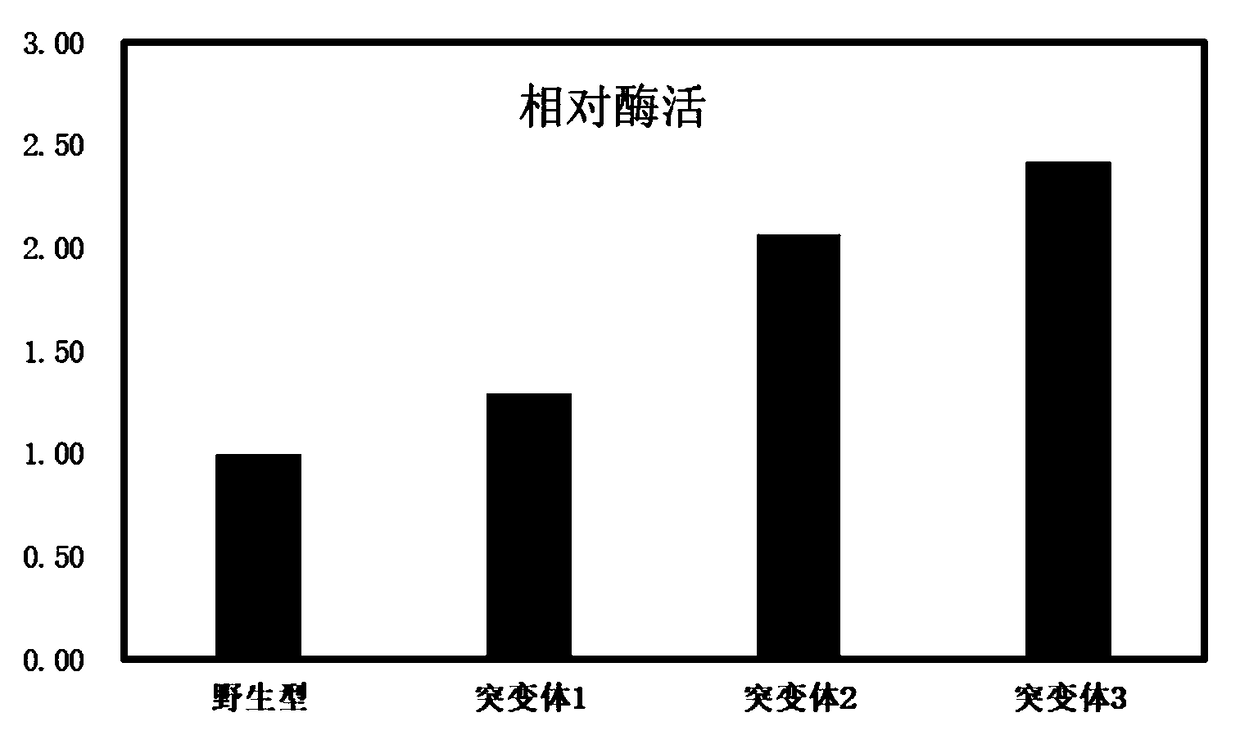
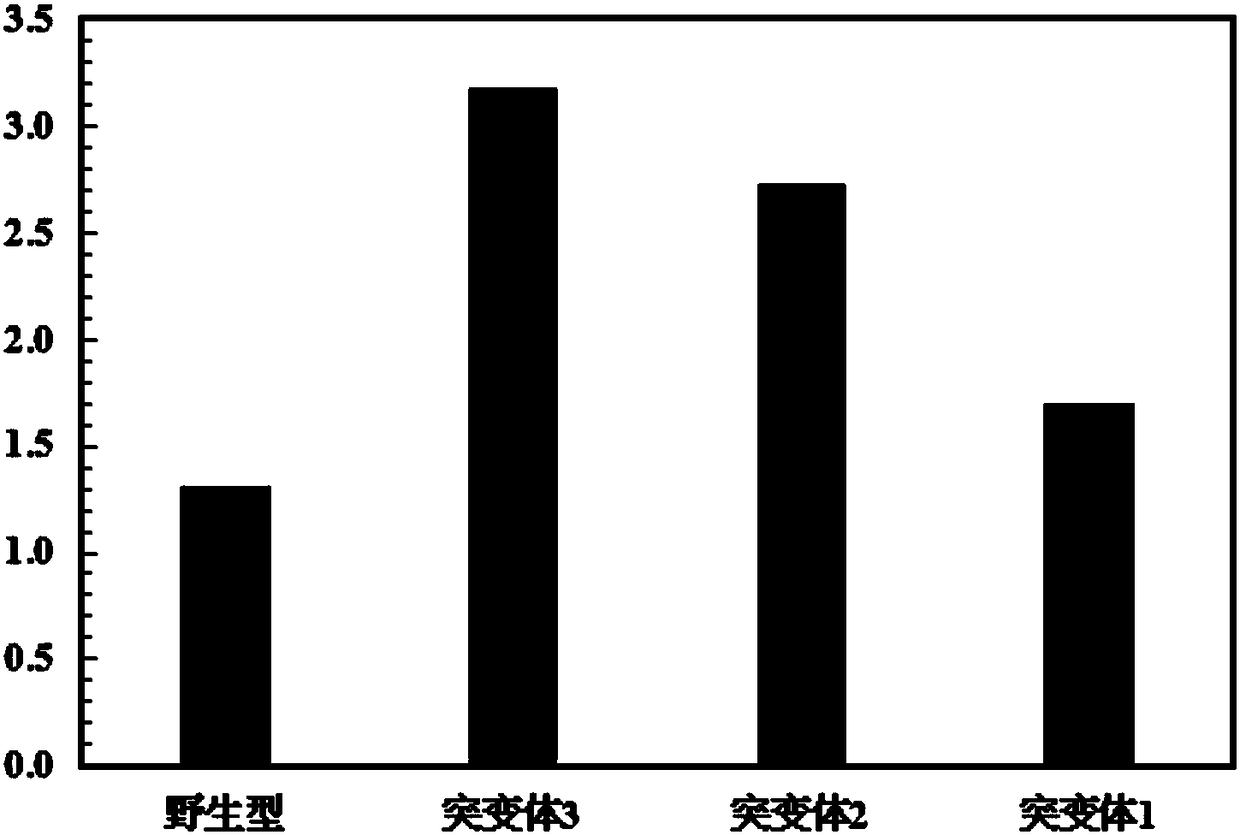
[0045] Embodiment 3: relative activity comparison
[0046] All PET membranes were washed in three consecutive steps: first in 1% SDS solution, then with ethanol, and finally with deionized water, before hydrolysis of the PET membranes with wild-type IsPETase and the respective mutant protein PETases. Each step lasts 30 minutes.
[0047] Hydrolysis of PET film: Take four PET films (1.5cm×1.0cm, about 36mg) and place them in a reaction containing 50μg wild-type IsPETase and three mutant protein PETases and 1.5ml 50mM Bicine-NaOH (pH8.5) respectively solution, reacted at 30°C for 48 hours. The aqueous solution was diluted with 1.0M NaOH, followed by heat treatment (50°C, 10 minutes) to terminate the reaction. The final products of each group were analyzed by reverse phase HPLC, the results are shown in figure 1 . The changes in the quality of PET films in each group are as follows: figure 2 (PET film mass change / mg).
[0048] figure 1 The results showed that compared with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com