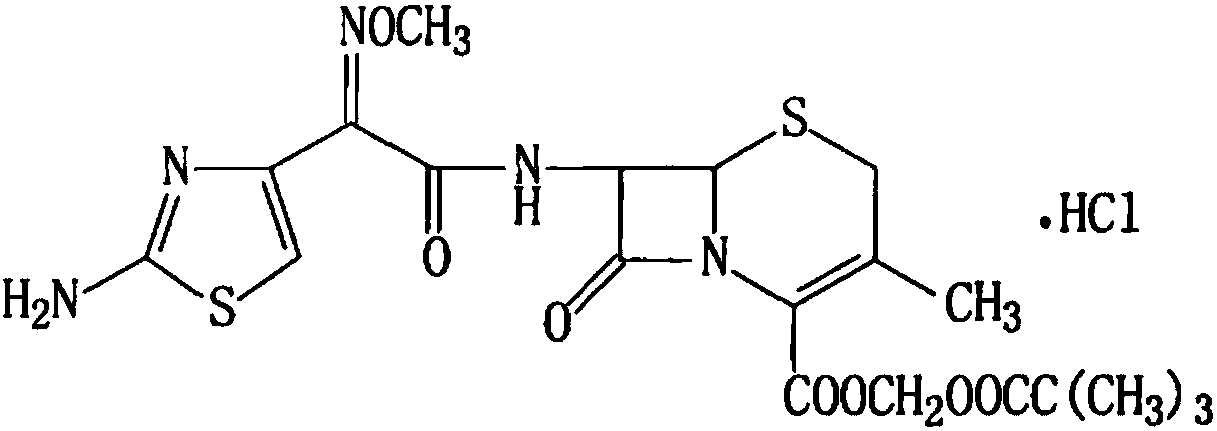
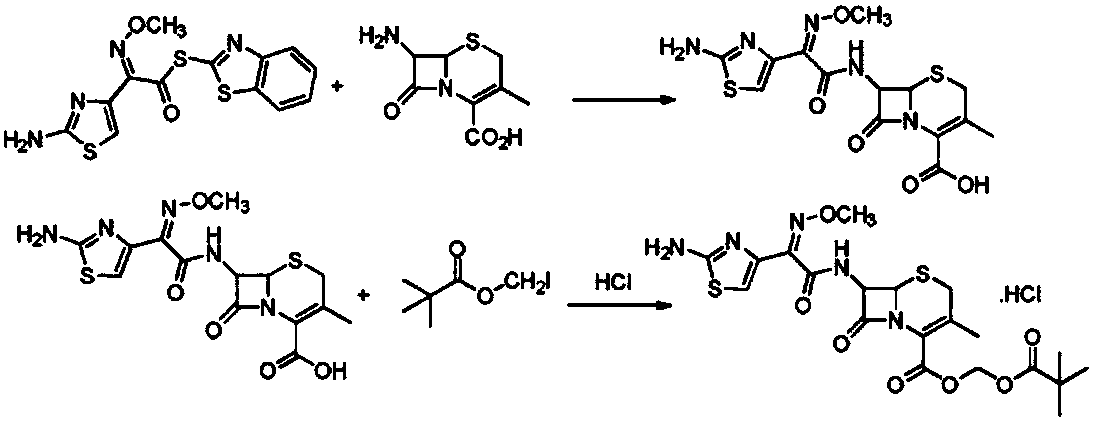
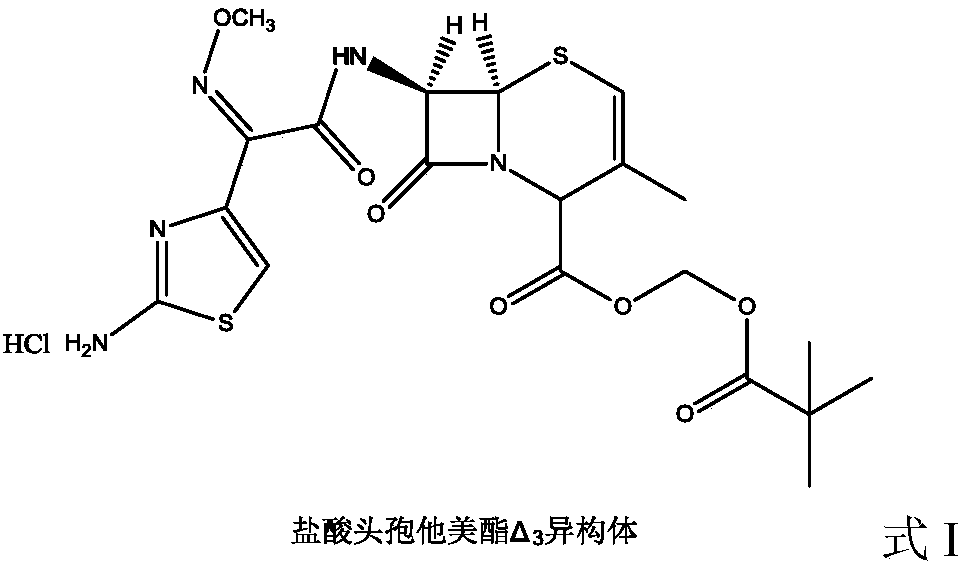
Cefetamet pivoxil hydrochloride preparation method
A technology of ceftametoxil hydrochloride and dilute hydrochloric acid, which is applied in the field of drug synthesis, can solve the problems of poor stability, easy deterioration, and low yield, and achieve the effects of mild reaction conditions, increased reactivity, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1) Prepare a trimethylaluminum hexane solution with a concentration of 2M at 5°C for later use; prepare 3-methyl-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0 ] oct-2-ene-2-carboxylic acid (7-ADCA) 4.71g was slowly added in 50mL of dichloromethane, fully stirred, then gradually added 12mL of a 2M trimethylaluminum hexane solution, stirred, in batches Add 4.59g of ethyl aminothioxamate, react at 35°C for 2 hours, monitor the completion of the reaction by TLC, cool to room temperature, slowly add 50mL of water to quench the reaction, let stand to separate layers; collect the water phase, adjust the pH to 2.0-2.5 with 6% hydrochloric acid , crystallized for 2.0 h, filtered, washed with 20 mL each of water and acetone successively, sucked dry, and dried in vacuum at 35°C to obtain 6.55 g of intermediate I, with an HPLC purity of 99.89% and a yield of 98.23%.
[0023] Step 2) Add 60mL of N-methylpyrrolidone and 3.33g of intermediate I to the reaction flask successively, stir,...
Embodiment 2
[0025] Step 1) Prepare a trimethylaluminum hexane solution with a concentration of 2M at 5°C for later use; prepare 3-methyl-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0 ] 4.28 g of oct-2-ene-2-carboxylic acid (7-ADCA) was slowly added to 50 mL of dichloromethane, fully stirred, and then gradually added 10 mL of a hexane solution of 2M trimethylaluminum, stirred, and batchwise Add 4.59g of ethyl aminothioxamate, react at 35°C for 2 hours, monitor the completion of the reaction by TLC, cool to room temperature, slowly add 50mL of water to quench the reaction, let stand to separate layers; collect the water phase, adjust the pH to 2.0-2.5 with 4% hydrochloric acid , crystallized for 2.0 h, filtered, washed with 20 mL of water and 20 mL of acetone successively, sucked dry, and dried in vacuum at 35° C. to obtain 6.12 g of intermediate I. The HPLC purity was 99.21%, and the yield was 91.23%.
[0026]Step 2) Add 50mL of N-methylpyrrolidone and 3.33g of intermediate I to the reaction fla...
Embodiment 3
[0028] Step 1) Prepare a trimethylaluminum hexane solution with a concentration of 2M at 5°C for later use; prepare 3-methyl-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0 ] Oct-2-ene-2-carboxylic acid (7-ADCA) 5.14g was slowly added to 50mL of dichloromethane, fully stirred, then gradually added 14mL of hexane solution of trimethylaluminum with a concentration of 2M, stirred, in batches Add 4.59g of ethyl aminothiaxamate, react at a temperature of 35°C for 2h, monitor the completion of the reaction by TLC, cool to room temperature, slowly add 50mL of water to quench the reaction, let stand to separate layers; collect the water phase, adjust the pH to 2.0-2.5 with 8% hydrochloric acid , crystallized for 2.0 h, filtered, washed with 20 mL of water and 20 mL of acetone in turn, sucked dry, and dried in vacuo at 35°C to obtain 6.44 g of Intermediate I with a HPLC purity of 99.74% and a yield of 96.37%.
[0029] Step 2) Add 60mL of N-methylpyrrolidone and 3.33g of intermediate I to the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com