Method for measuring methanesulfonate by virtue of derivatization HPLC-UV method
A mesylate and derivatization technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of lower detection sensitivity, tailing, low sensitivity, etc., and achieve good linear relationship, good specificity, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] 3. Preparation of Solutions
[0045] Methyl methanesulfonate stock solution: take about 10 mg of methyl methanesulfonate, accurately weigh it, put it in a 10 ml measuring flask, dissolve it with acetonitrile and dilute to the mark, and shake well to obtain 1 mg / ml.
[0046] Ethyl methanesulfonate stock solution: take about 10 mg of ethyl methanesulfonate, accurately weigh it, put it in a 10 ml measuring flask, dissolve it with acetonitrile and dilute it to the mark, and shake well to obtain 1 mg / ml.
[0047] Serial mesylate mixed stock solution: Precisely measure equal amounts of methyl methanesulfonate stock solution and ethyl methanesulfonate stock solution, place them in a 10ml volumetric flask, add acetonitrile to dilute to the mark, and dilute to contain the same concentration of methyl methanesulfonate. A series of mixed stock solutions of methyl sulfonate and ethyl methanesulfonate (concentrations 5, 10, 20, 30, 40, 50 μg / ml).
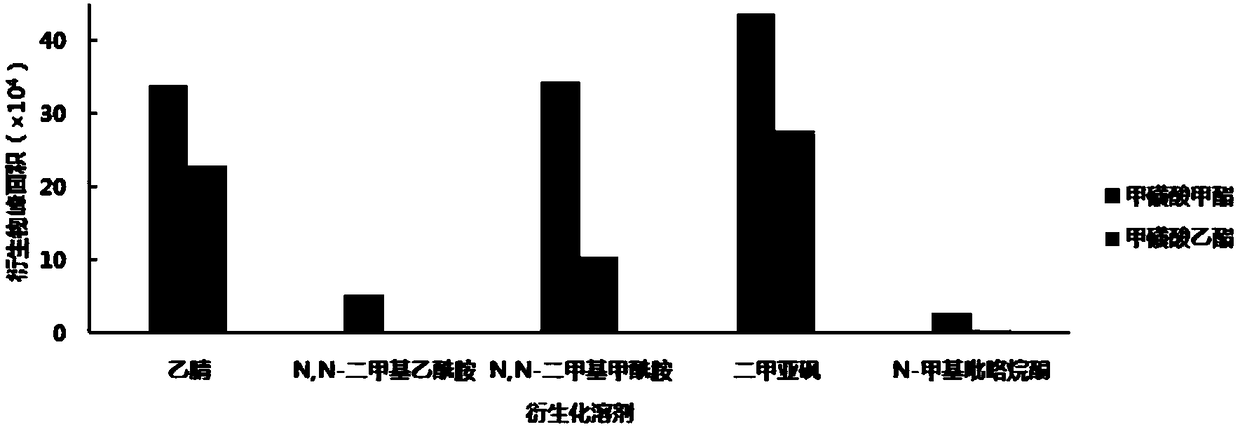
[0048] 2-Naphthalene thiophenol d...
Embodiment 1
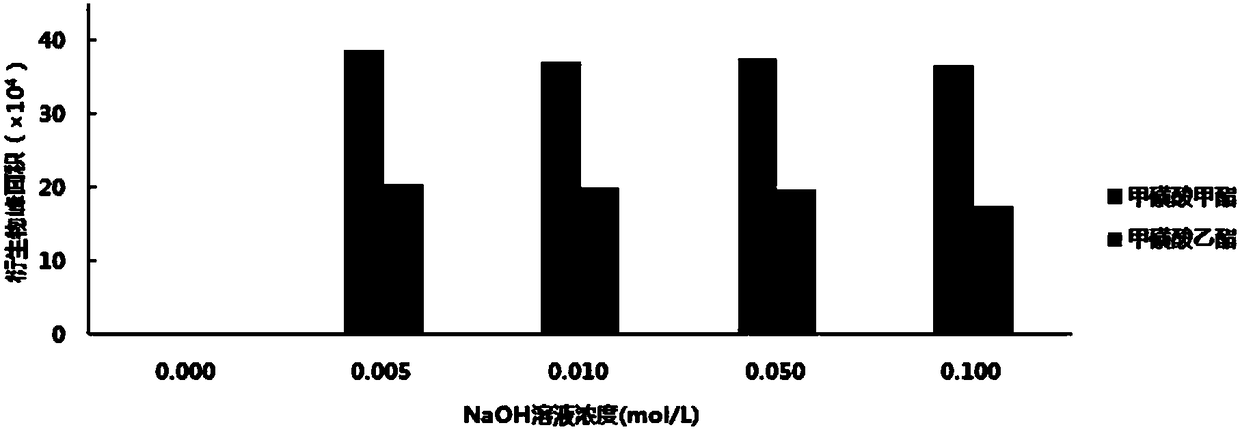
[0053] Precisely measure 0.1ml of 50μg / ml mesylate mixed stock solution, place it in a 5ml volumetric flask, add 1.0ml of 1.0mg / ml 2-naphthalene thiol derivatization solution and 0.10mol / L NaOH solution in turn 0.5ml, diluted to the mark with acetonitrile, shake well, react at 80°C for 2h, let cool to room temperature, centrifuge at 10000rpm for 5min, take 20μl of the supernatant and inject it into HPLC-DAD analysis.
[0054] HPLC-DAD conditions: instrument conditions are the same as described in 1; chromatographic conditions are the same as described in 4; DAD scans at 200-400 nm.
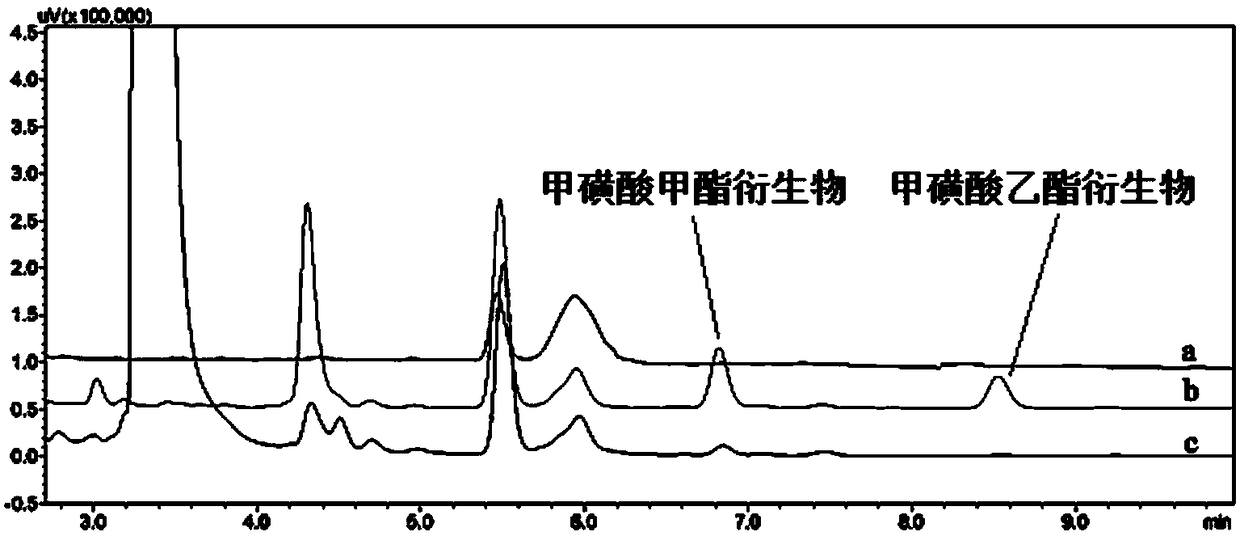
[0055] The maximum absorption wavelength of the methyl methanesulfonate product is 252 nm, and the maximum absorption wavelength of the ethyl methanesulfonate product is 253 nm, and the maximum absorption wavelength of the ethyl methanesulfonate product is selected as the detection wavelength.
Embodiment 2
[0057] Precisely measure 6 parts, each 0.10ml of the serial mesylate mixed stock solution, place them in 6 5ml volumetric flasks, add 1.0ml and 0.50mol / ml of 5.0mg / ml 2-naphthalene thiophenol derivatization solution 0.5ml of L NaOH solution, dilute to the mark with acetonitrile, shake well, prepare a solution of 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 μg / ml of mesylate, respectively, react at 80 °C for 2 h, and let it cool until At room temperature, centrifuge at 10,000 rpm for 5 min, and take 20 μl of the supernatant and inject it into HPLC-UV analysis.
[0058] Take about 100 mg of imatinib mesylate, accurately weigh it, place it in a 10 ml measuring flask, dissolve it with water-acetonitrile solution (10:90, v / v) and dilute it to the mark. Precisely measure 0.5ml, put it in a 5ml measuring bottle, add 1.0ml of 5.0mg / ml 2-thionaphthalene derivatized solution and 0.5ml of 0.50mol / L NaOH solution, dilute with acetonitrile to the mark, shake well, put it in The reaction was carried out a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com