Citral diborneol-based acetal derivative and preparation method and application thereof
A technology of citral dimethyl acetal and citral, which is applied in the field of citral dibornyl acetal derivatives and their preparation, can solve the problems of strong volatility, low efficacy, poor solubility and the like, and achieves mild reaction conditions , low difficulty, stable derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
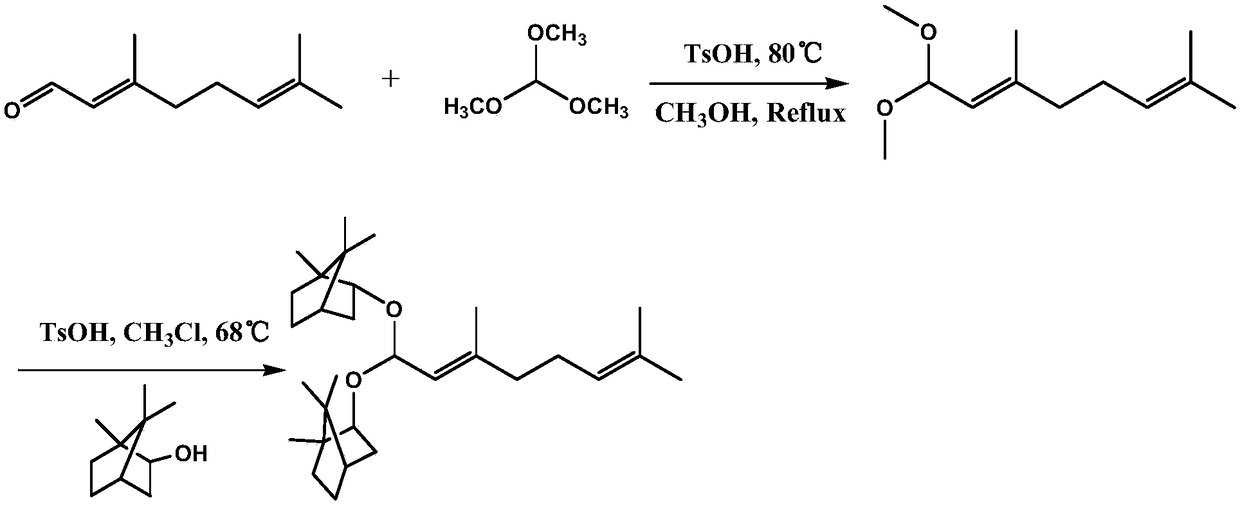
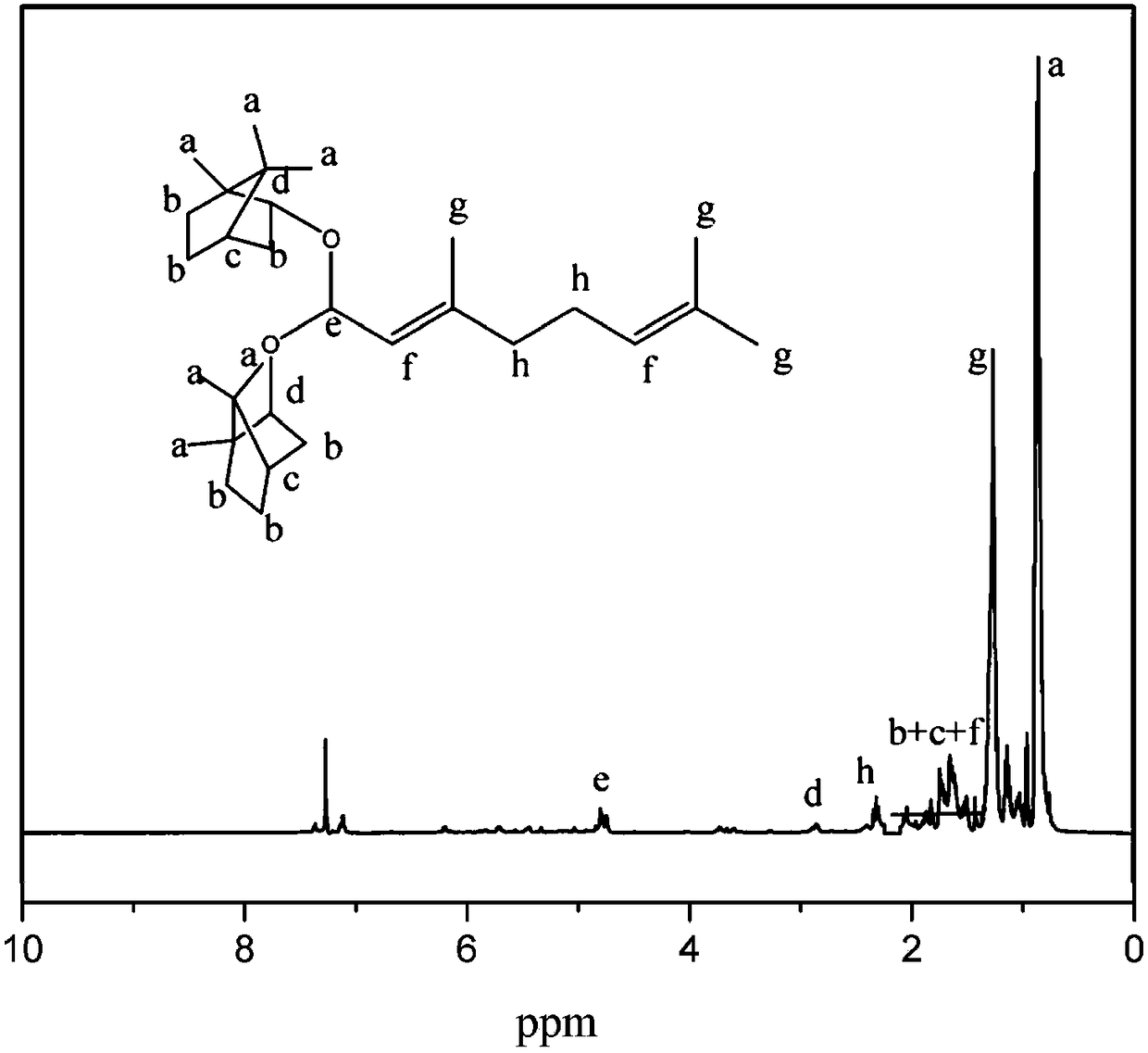
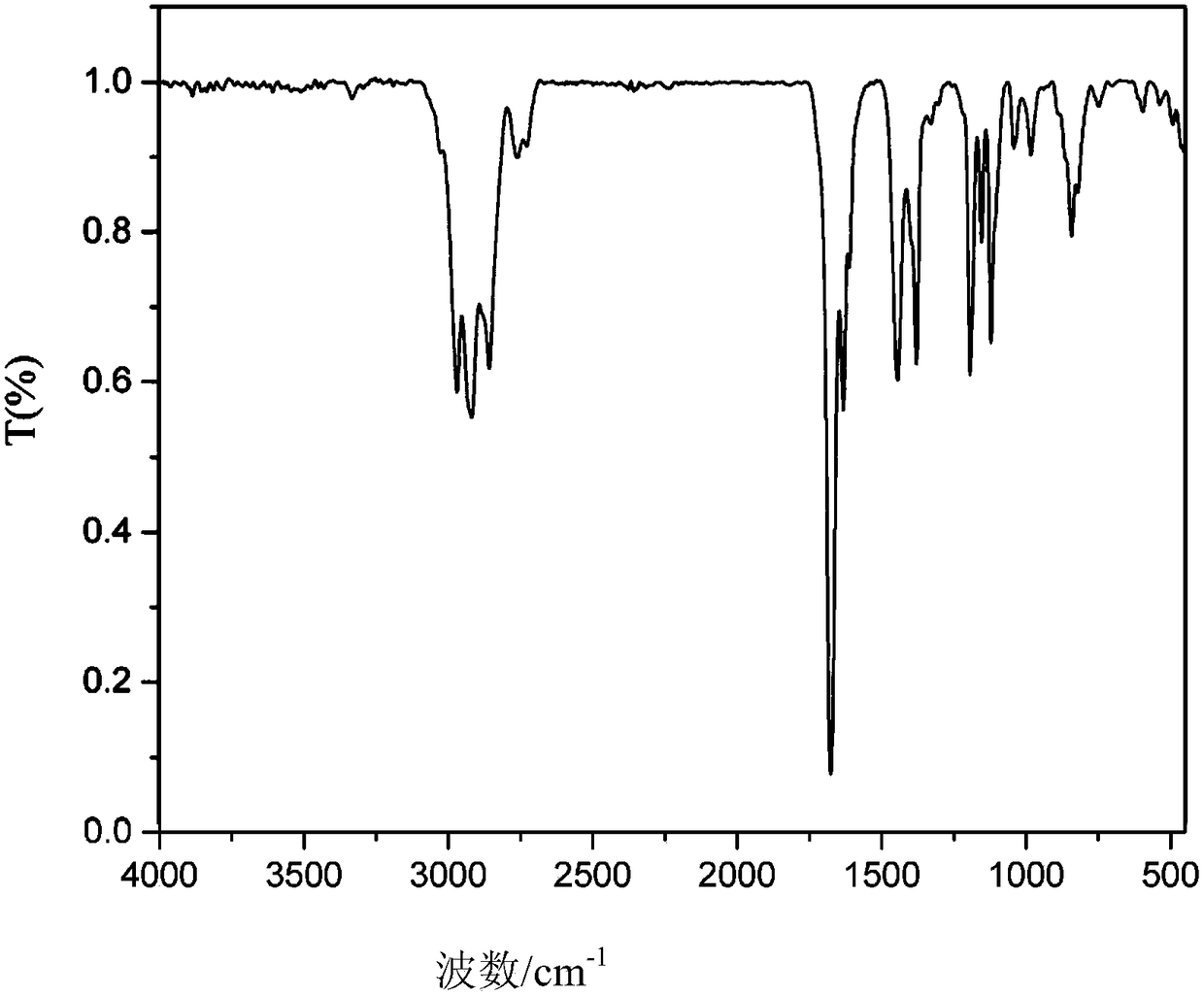
[0058] A kind of preparation of citral dibornyl acetal derivatives, such as figure 1 shown, including the following steps:
[0059] (1) At room temperature, take by weighing citral 7.6120g (0.05mol, molecular weight 152.23), trimethyl orthoformate 21.2240g (0.20mol, molecular weight 106.12) in a dry clean round bottom flask, dissolve in 50mL of methanol, Add 28.56mg of p-toluenesulfonic acid, and the reaction system is refluxed at 80°C and 800r / min for 6 hours;
[0060] (2) Add saturated sodium bicarbonate aqueous solution to the reaction system in batches to pH=7-8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 30mL dichloromethane, collect the organic layer, add 9.4522g anhydrous sulfuric acid Sodium dehydration for 6 hours;
[0061] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and use a silica gel column with an average particle size of 48 μm for analysis: first, dissolve the silica g...
Embodiment 2
[0071] (1) At room temperature, weigh 3.8221g (0.025mol) of citral and 10.3421g (0.097mol) of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 40mL of methanol, and add 15.21mg of p-toluenesulfonate acid, the reaction system was refluxed at 75°C and 800r / min for 4 hours.
[0072] (2) Add saturated aqueous sodium bicarbonate solution to pH = 7-8 in batches to the reaction system, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 30 mL of dichloromethane, collect the organic layer, add 5.3214 g of anhydrous sulfuric acid Sodium dehydration for 4 hours.
[0073] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and use a silica gel column with an average particle diameter of 37 μm for analysis. : 1) flushing with eluent, wash out the impurities with low polarity first, and then elute with the solution obtained by mixing sherwood oil and ethyl acetate at a volume ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com