A kind of chitosan/insulin nano sustained-release transdermal preparation and preparation method thereof
A nano-sustained-release, insulin technology, applied in pharmaceutical formulations, peptide/protein components, medical preparations with non-active ingredients, etc., can solve the problems of physiological pain, high price, and frequent injections of insulin injection, and avoid physiological and psychological problems. Pain, improve solubility, and reduce the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A: Weigh 0.1ml of glycerin, 200mg of hydroxymethylcellulose, 1g of mannitol, 0.4g of propylene glycol, 5mg of carbomer, and 150mg of sodium acrylate. Weigh 5.5538 mg of insulin and dissolve it in 1 ml of methanol to form a drug-containing organic solution. 11.1076 mg chitosan was dissolved in 1 mL 1wt% acetic acid, and 2 mg sodium tripolyphosphate (TPP) was added to chitosan acetic acid solution to make chitosan nano solution.
[0035] B: Dissolve the glycerin, hydroxymethylcellulose, mannitol, propylene glycol, carbomer, and sodium acrylate weighed in step A in 5ml of water, and at the same time take 100 μL insulin methanol solution and 250 μL chitosan nano solution and mix evenly, Add it dropwise to the hydrophilic excipient, stir at room temperature for 1 hour, then add the penetration enhancer, matrix and pressure-sensitive adhesive and mix well to obtain the drug-loaded layer;
[0036] D: Evenly coat the drug-loaded layer on the backing layer, cover with a protect...
Embodiment 2
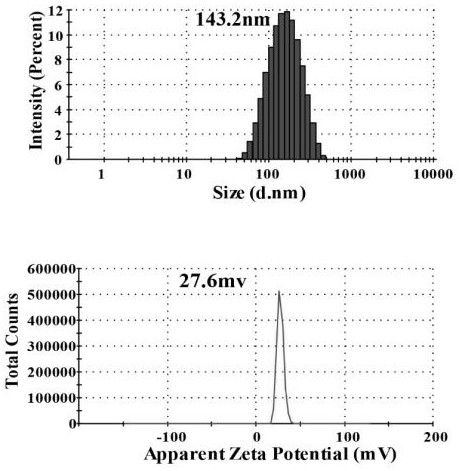
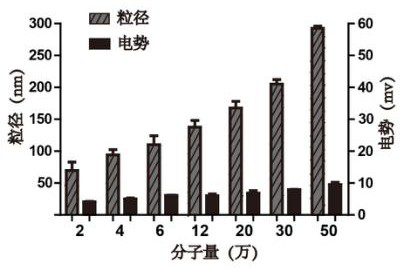
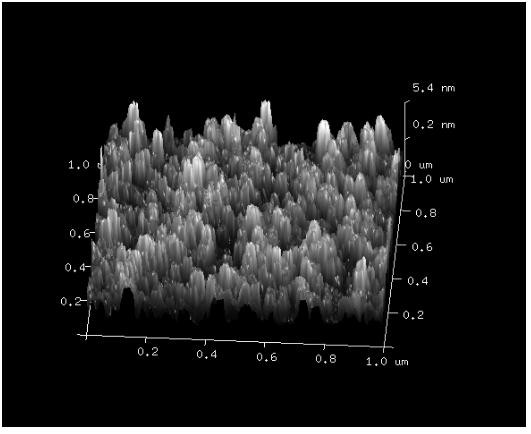
[0038] After taking 500 μL of insulin methanol solution and 750 μL of chitosan nano-water solution and mixing them evenly, use a Malvern particle size analyzer to measure the particle size of the nano-solution, such as figure 1 shown. At the same time, chitosan with different molecular weights was used to entrap insulin, and its particle size was measured, such as figure 2 As shown, it can be seen that the smaller the molecular weight of chitosan, the smaller the particle size of chitosan / insulin nanoparticles. And take atomic force images of chitosan / insulin nanoparticles with better particle size, such as image 3 shown.
Embodiment 3
[0040] Place 1ml of the drug-loaded layer in a dialysis bag with a molecular weight cut-off of 10,000, and at the same time place the dialysis bag in 30 ml of PBS solution (NaCl, 137 mM; KCl, 2.7 mM, Na 2 HPO 4 ,10 mM; KH 2 PO 4 , 2 mM; pH 7.4), glucose was added to the buffer solution to achieve a higher blood glucose concentration in the human body, and then the PBS buffer solution was placed in a constant temperature shaker at 37°C with a speed of 233min / r. Take a sample of 500 μl, and add 500 μl of PBS with a higher blood glucose concentration at the same time. Adopt ultraviolet spectrophotometry to detect wavelength 276nm, calculate cumulative amount, such as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com