CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cell cryopreservation liquid for direct venous retransfusion and preparation method and application of CAR-T cell cryopreservation liquid
A cryopreservation and cell technology, applied in the field of biotechnology applications and medicine, can solve the problems of cumbersome cryopreservation process and subsequent application procedures, unsuitable for large-scale production, unclear composition of cryopreservation solution, etc., and achieve the maintenance of immune cell activity. The effect is good, the quantity and source of raw materials are not limited, and the effect of ensuring safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 28
[0045] Example 1-Example 28: Preparation of cryopreservation solution
[0046] raw material
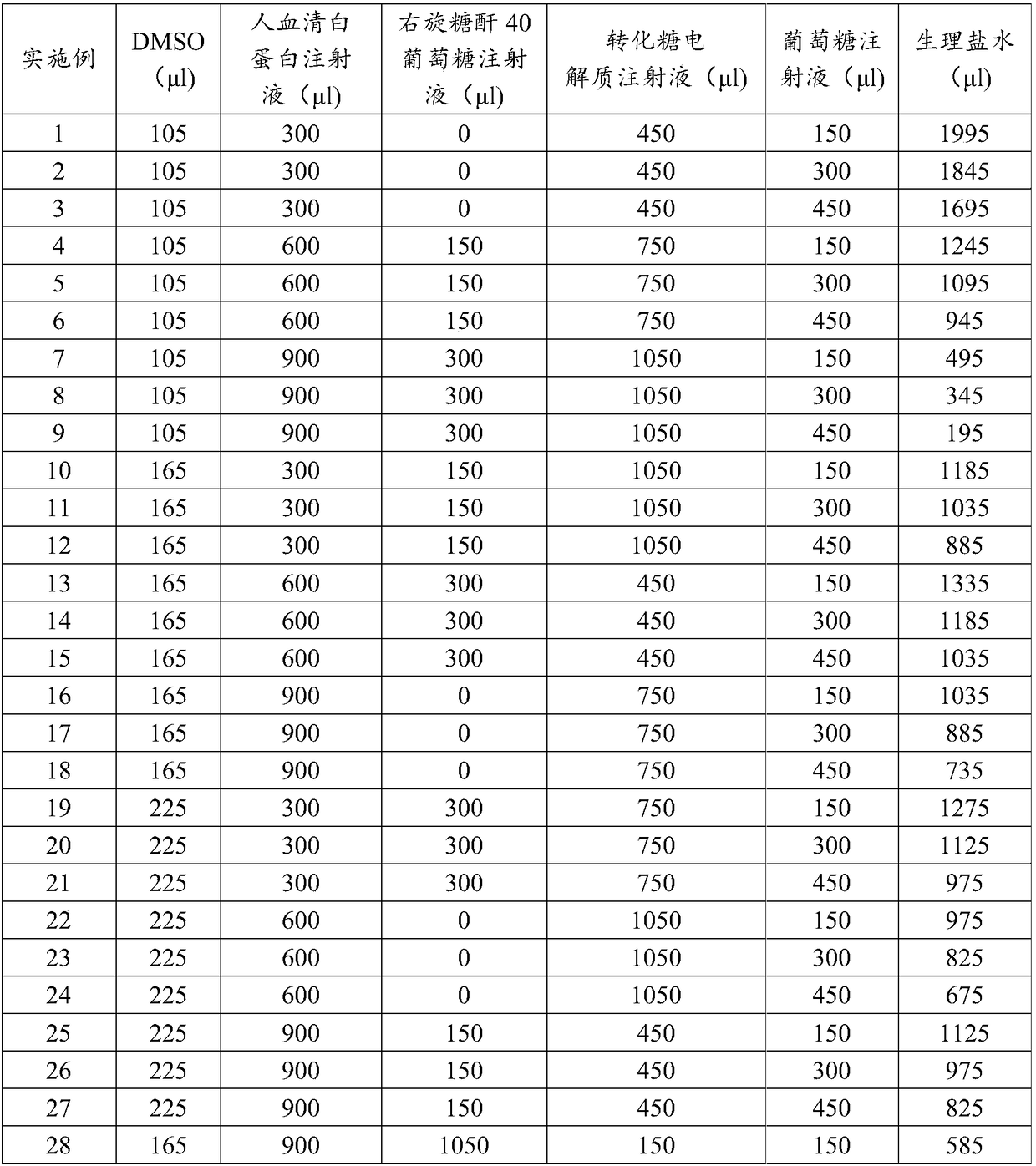
[0047] Table 1: Raw material ratio of frozen storage solution of embodiment 1-embodiment 28
[0048]
[0049] Embodiments 1-27 are the orthogonal experimental proportions of the frozen storage liquid raw materials, and the analysis of the orthogonal experimental results shows that embodiment 28 is the best proportion of the frozen storage liquid raw materials.
[0050] 1. Preparation method
[0051] Accurately weigh various raw materials according to the amount in Table 1 to obtain a frozen storage solution.
Embodiment 29
[0052] Example 29: Cryopreservation preparation of CAR-T cells
[0053] 2. Materials
[0054] 1) CAR-T cells, prepared according to the method of Chinese patent application 201610014730.6 (CAR-T cells from other sources can also be used).
[0055] 2) Freeze storage solution, prepared according to the ratio of Example 28.
[0056] 3. Method
[0057] 1) CAR-T cell isolation
[0058] a) Mix the CAR-T cells cultured for 10 days, transfer to a 50ml centrifuge tube, centrifuge at 500g for 6min, and discard the supernatant.
[0059] b) Resuspend the centrifuged cell mass with 10ml of 0.9% sodium chloride injection, confirm that the cell mass is completely broken, add 0.9% sodium chloride injection to 25ml, rest on the magnetic stand for 2min, pass through the magnetic force The magnetism of the frame realizes the separation of cells and magnetic beads.
[0060] c) Transfer the cell suspension to a new 50ml centrifuge tube with a pipette, and repeat the demagnetization operation ...
Embodiment 30
[0067] Example 30: Inspection of cryopreserved preparations of CAR-T cells
[0068] 1. Materials
[0069] The CAR-T cell cryopreservation preparation obtained in Example 30
[0070] Trypan blue staining reagent;
[0071] Other commercial freezing solutions: CTS TM Medium, purchased from Yingwei Jieji (Shanghai) Trading Co., Ltd.;
[0072] Tumor cell line: U266 cell line, purchased from China Center for Type Culture Collection.
[0073] 2. Method
[0074] 1) Cell recovery: Take out the cell cryopreservation bag from the liquid nitrogen tank, and put it into a 37°C water bath to thaw quickly; after the cell mixture in the cryopreservation bag is completely dissolved, slowly add the cell mixture into a certain volume of T In a centrifuge tube of complete cell culture medium, mix well; centrifuge at 500g for 6min, discard the supernatant, and set aside.
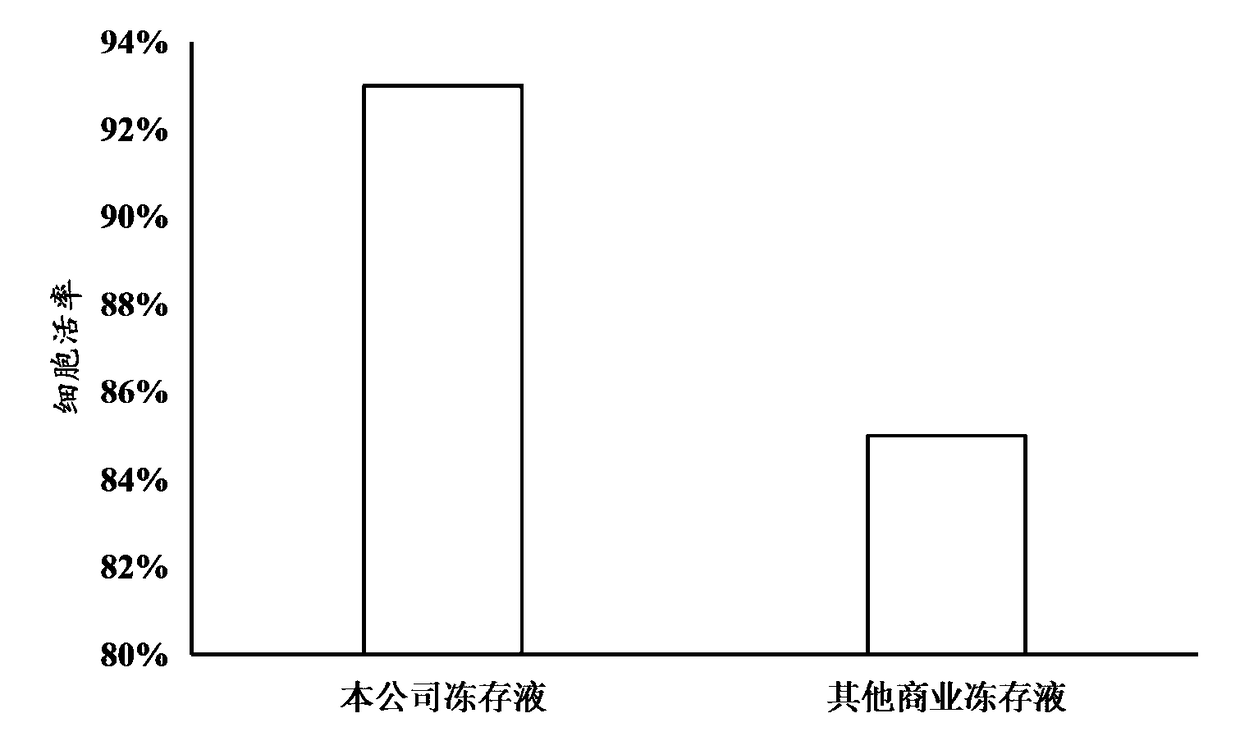
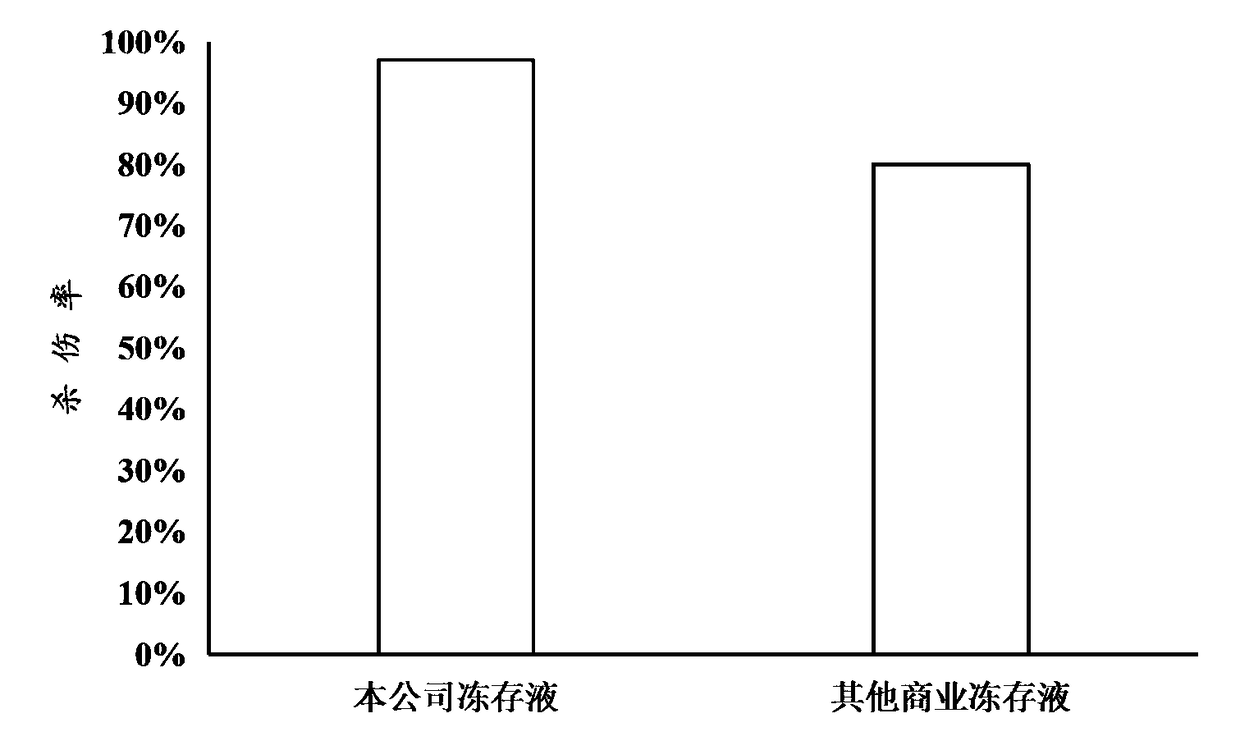
[0075] 2) Determination of cell viability
[0076] After the CAR-T cells were prepared in cryopreservation solution, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com