Application of rotenone in preparing medicine for treating diabetic nephropathy
A technology for diabetic nephropathy and rotenone, which is applied in the field of application of rotenone in the preparation of drugs for diabetic nephropathy, and can solve problems such as differences in treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The safety evaluation of embodiment 1 low-dose rotenone (100ppm is mixed and taken in feed)
[0029] 1 Experimental materials
[0030] The C57BL / 6 species mice used in the present invention were purchased from the Experimental Animal Center of Nanjing Medical University;
[0031] The present invention uses rotenone (Rotenone, ROT) purchased from Sigma-Aldrich Company. 100ppm rotenone feed (100mg rotenone was uniformly mixed into 400kg feed powder, mixed with 600mL agar powder aqueous solution (2%, m / v) sugar to form); the feed used by the mice in the control group was a drug-free feed prepared in the same way.
[0032] 2 Experimental methods
[0033] 2.1 Animal administration method
[0034] Thirty male C57BL / 6 mice (7 weeks old, body weight 20-24g) were fed adaptively for 1 week and randomly divided into Ctrl group (n=7) and ROT group (n=7). Before the experiment, the animals had free access to food and maintained a circadian rhythm of 12 hours of light and 12 hour...
Embodiment 2
[0039] Example 2 Rotenone Improves Diuretic Symptoms, Increased Kidney Weight and Glomerular Hypertrophy in Diabetic Nephropathy Mice
[0040] 1 Experimental materials
[0041] Streptozocin (STZ) and sodium citrate used in the present invention were purchased from Sigma-Aldrich Company. STZ is dissolved in sodium citrate solution (pH=4.0), ready for immediate use. Other biological materials and chemical materials are the same as in Example 1.
[0042] 2 Experimental methods
[0043] 2.1 Establishment of mouse diabetic nephropathy model and administration method of rotenone
[0044] Thirty male C57BL / 6 mice (7 weeks old, body weight 20-24g) were fed adaptively for 1 week and then underwent unilateral nephrectomy. The specific method is: take a midline incision in the abdomen of the mouse, cut the muscle layer along the linea alba, enter the abdominal cavity, push the intestinal tube to the opposite side, expose the kidney and blood vessels on one side, fully free the blood ...
Embodiment 3
[0052] Example 3 Rotenone improves the level of kidney damage in mice with diabetic nephropathy
[0053] 1 Experimental materials
[0054] The urine microalbumin detection kit used in the present invention was purchased from Bethyl Laboratories; other biological materials and chemical materials were the same as in Example 1.
[0055] 2 Experimental methods
[0056] 2.1 Serum biochemical detection
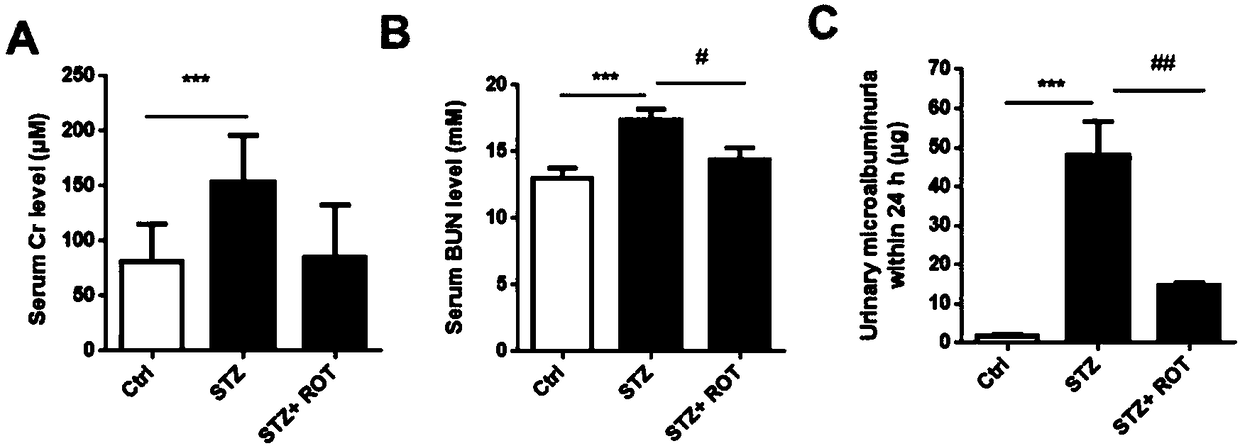
[0057] Whole blood samples from mice in each group were collected, left at room temperature for 1-2 hours, centrifuged at 4000rpm for 10 minutes, and the supernatant was transferred to obtain serum samples for determination of serum creatinine (Cr) and blood urea nitrogen (BUN) levels.
[0058] 2.2 Detection of urinary microalbumin
[0059] Take the urine of the mice in each group at the 4th week after the start of treatment in the STZ+ROT group, dilute it according to urine:PBS=1:200, and perform quantitative detection according to the instructions.
[0060] 24h urinary microal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com