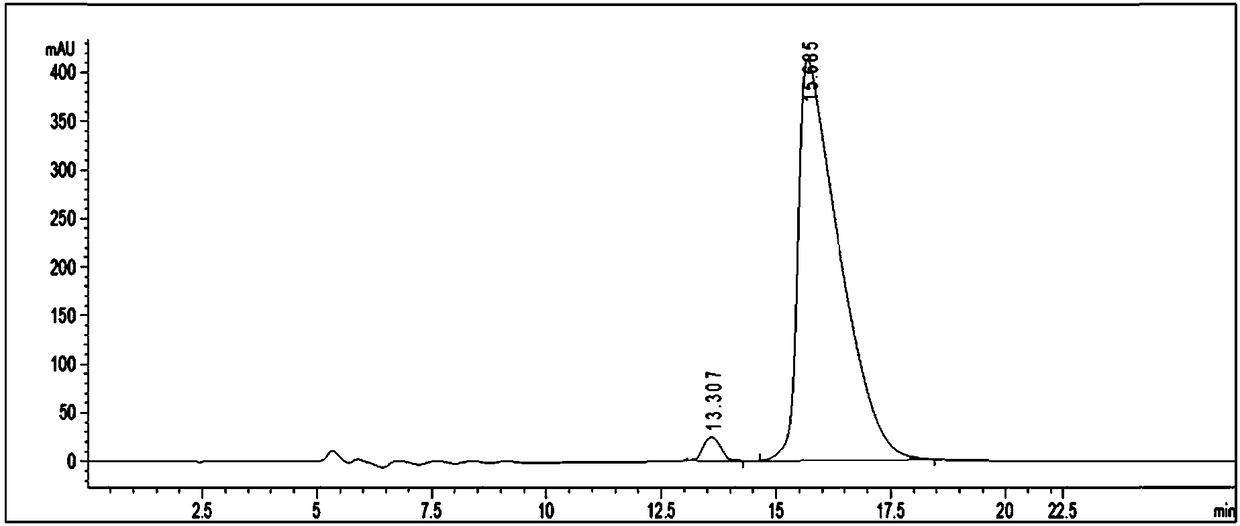
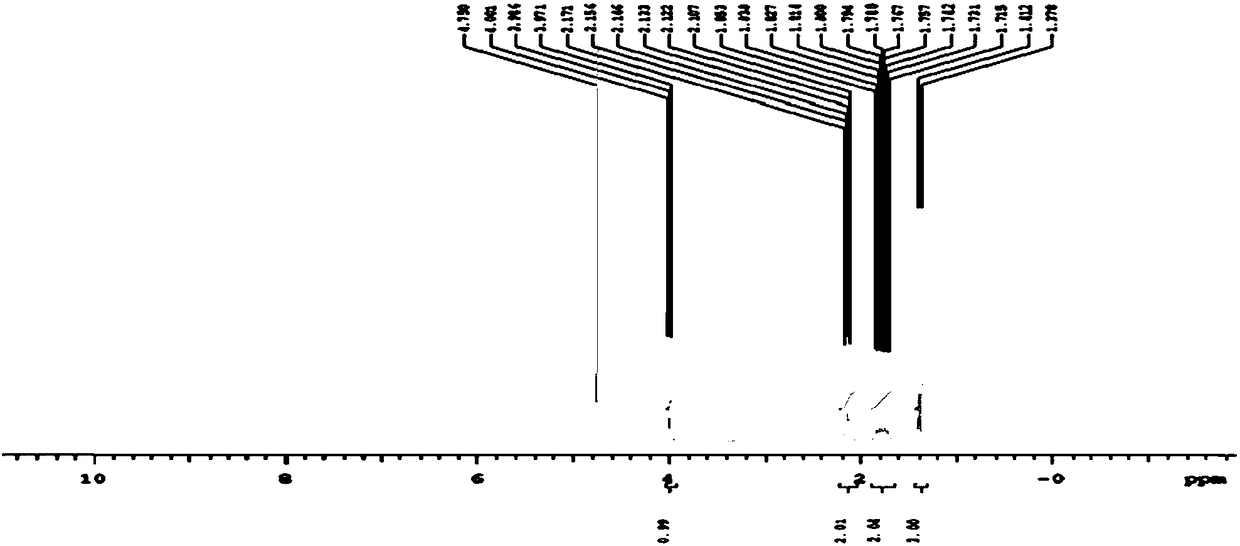
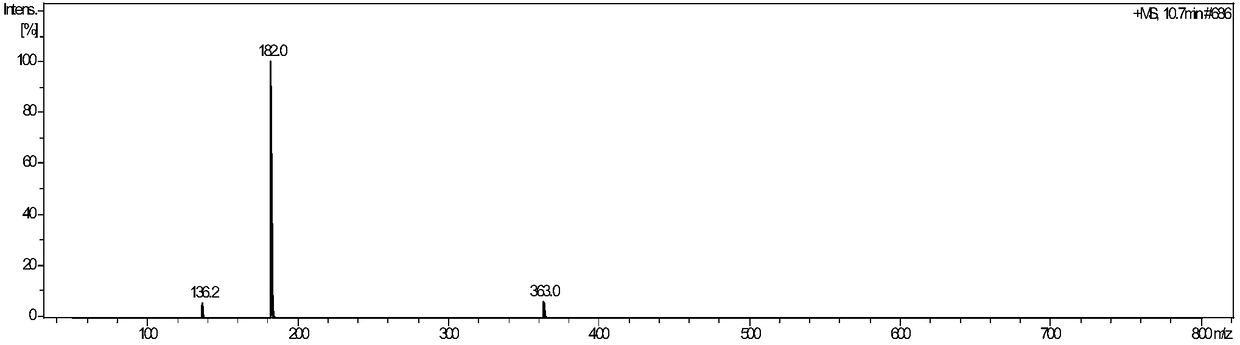
Preparation method of fine glufosinate
An essence glufosinate-ammonium and reaction technology, applied in the field of organic compound synthesis, can solve the problems of high cost, complicated operation, low yield and the like, and achieve the effects of reducing production cost, simple process and improving total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthetic method of essence glufosinate-ammonium described in the present embodiment comprises the steps:
[0064] (1) Synthesis of Compound III: Add 110g (0.92mol) of L-homoserine, 300ml toluene, and 2g (0.012mol) of p-toluenesulfonic acid to a 2L three-necked flask, connect the azeotropic dehydration device, stir evenly, and slowly heat to 108°C, carry out azeotropic dehydration reaction for 12h, then cool to 80°C, slowly add 142.8g (1.2mol) of thionyl chloride dropwise, stir and react for 4h, cool to room temperature after the reaction, filter, and take the filter cake with toluene (50mL ×3 times), water (50mL×5 times), and dried to obtain 98.5g of white solid powder, which is the desired compound of formula (Ⅲ), that is, L-3,6-bis(2-chloroethyl) -2,5-diketopiperazine, the calculated yield is 88.7%;
[0065] (2) Synthesis of Compound IV: Under nitrogen protection, add 98.5 g (0.408 mol) of the obtained L-3,6-bis(2-chloroethyl)-2,5-diketopiperazine into a 2L thre...
Embodiment 2
[0070] The synthetic method of essence glufosinate-ammonium described in the present embodiment comprises the steps:
[0071] (1) Synthesis of compound III: Add 59.5g (0.5mol) of L-homoserine, 200ml of benzene, and 1.58g (0.01mol) of benzenesulfonic acid into a 1000mL three-neck flask, connect the azeotropic dehydration device, stir evenly, and heat slowly To 80°C, azeotropic dehydration reaction for 30h; then cool to 60°C, add 1.6g (0.02mol) of pyridine, slowly drop in 98.9g (0.5mol) of diphosgene, stir for 10h, cool to room temperature, filter, filter cake Wash with benzene (30mL×3 times), water (30mL×5 times), and dry the filter cake to obtain 52.7g of white solid powder compound III, namely L-3,6-bis(2-chloroethyl)-2, 5-diketopiperazine, calculate its productive rate 86.5%;
[0072] (2) Synthesis of compound IV: with step (2) in Example 1;
[0073] (3) Synthesis of essence glufosinate-ammonium: same as step (3) in Example 1.
Embodiment 3
[0075] The synthetic method of essence glufosinate-ammonium described in the present embodiment comprises the steps:
[0076] (1) Synthesis of Compound III: Add 71.4g (0.6mol) of L-homoserine, 200ml of xylene, and 1.6g (0.01mol) of p-benzenesulfonic acid into a 1000mL three-neck flask, connect the azeotropic dehydration device, and stir evenly. Slowly heat to 140°C, azeotropic dehydration reaction for 8h; then cool to 80°C, slowly drop in 107g (0.9mol) of thionyl chloride, stir for 4h, cool to room temperature; filter, filter cake with toluene (30mL×3 times ), water (30mL×5 times), and the filter cake was dried to obtain 64.1g of white solid powder compound III, namely L-3,6-bis(2-chloroethyl)-2,5-diketopiperazine, Calculate the yield of 87.4%;
[0077] (2) Synthesis of compound IV: with step (2) in Example 1;
[0078] (3) Synthesis of essence glufosinate-ammonium: same as step (3) in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com