A kind of electrochemical preparation method of n-(3,5-dimethyl-4-hydroxyphenyl) acetamide
A technology of hydroxyphenyl and dimethylphenol, which is applied in the field of electrochemical organic synthesis, can solve the problems of high energy consumption, and achieve the effects of good reaction selectivity, simple raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
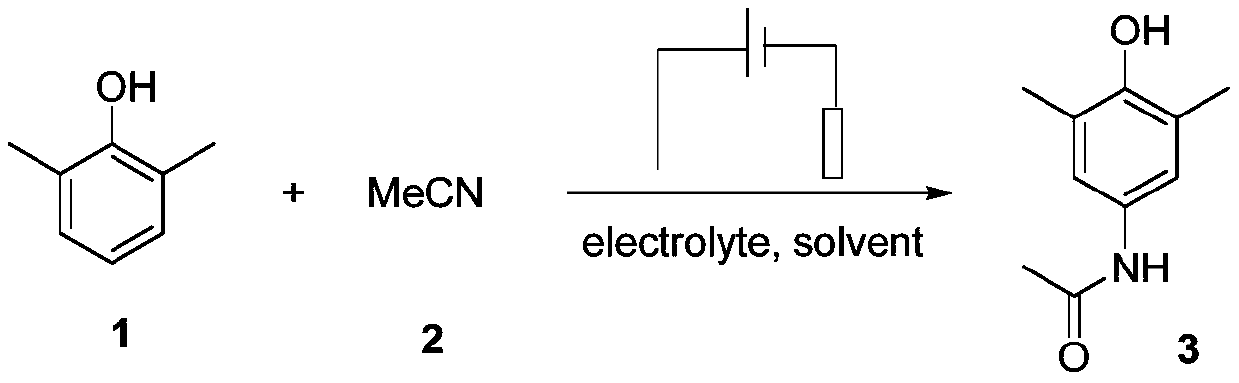
[0030] With metal platinum wire as anode and platinum sheet as cathode, add 0.5mmol sodium fluoroborate, 0.03mmol nickel chloride, 0.3mmol 2,6-dimethylphenol, 0.15mmol trifluoroacetic acid, 0.075mmol fluorine Potassium chloride, 200uL tert-butanol, 5mL acetonitrile, magnetic stirrer, cover the lid, turn on the power, adjust the current to 5mA, and electrolyze at room temperature for 5h. After the reaction, the reaction liquid was extracted with ethyl acetate, and the corresponding product 3 was obtained after separation and purification, and the yield of the product 3 was 73%. The reaction scheme of the present embodiment is as follows:
[0031]
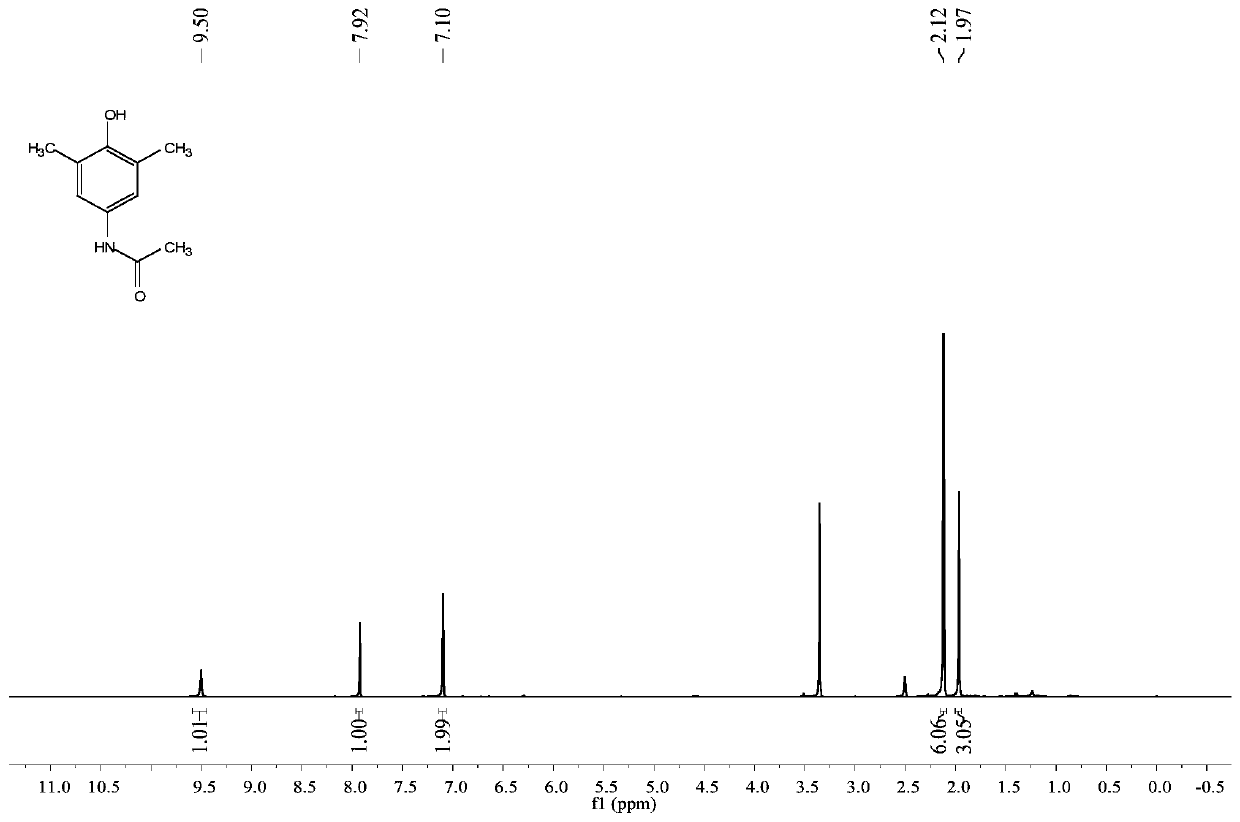
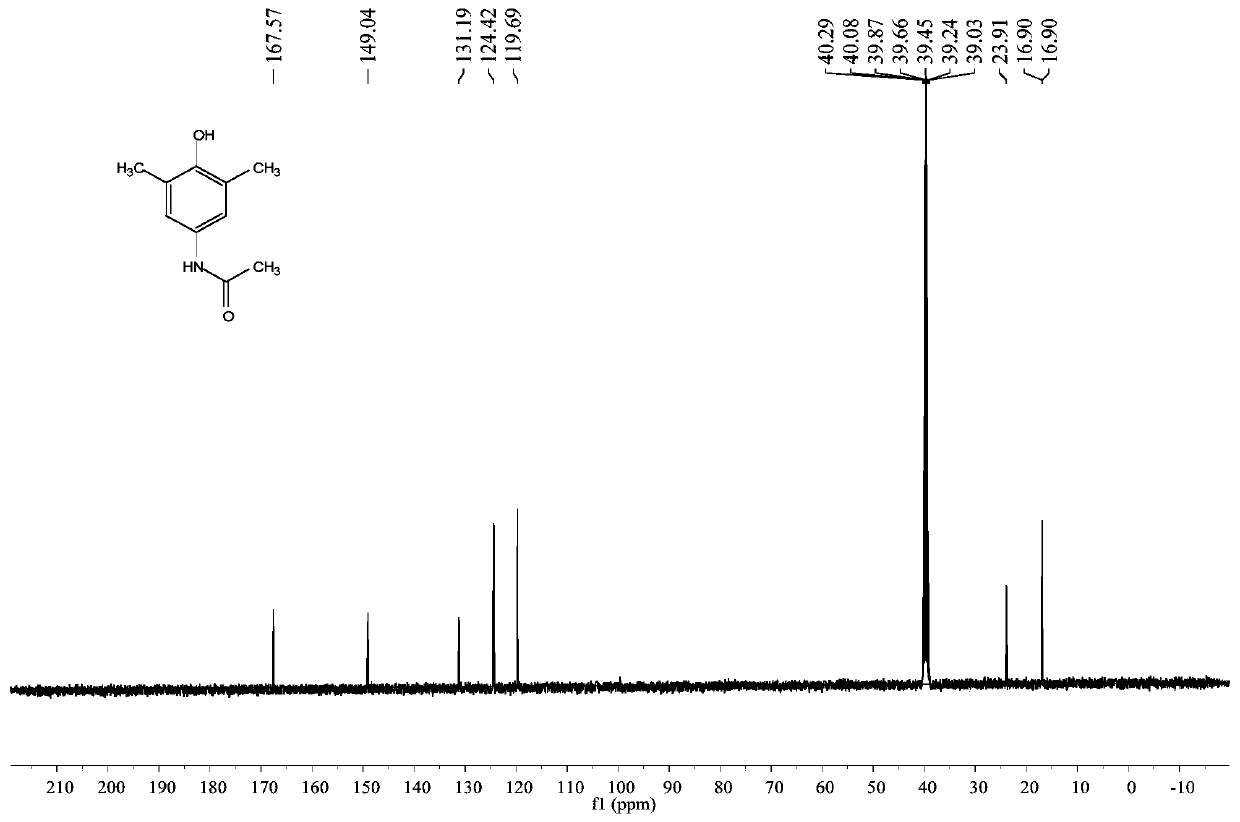
[0032] The proton nuclear magnetic resonance spectrum figure of present embodiment product is as figure 1 Shown: 1 HNMR (400MHz, DMSO-d 6 ): δ (ppm) 9.50 (s, 1H), 7.92 (s, 1H), 7.10 (s, 2H), 2.12 (s, 6H), 1.97 (s, 3H); figure 2 Shown: 13 CNMR (100MHz, DMSO-d 6 ): δ (ppm) 167.6, 149.0, 131.2, 124.4, 119.7, 23.9, 16.9.
[00...
Embodiment 2
[0035] With metal platinum wire as anode and platinum sheet as cathode, add 1mmol sodium fluoroborate, 0.06mmol nickel chloride, 0.3mmol 2,6-dimethylphenol, 0.18mmol trifluoroacetic acid, 0.0825mmol fluoride Potassium, 200uL tert-butanol, 5mL acetonitrile, magnetic stirrer, cover the lid, turn on the power, adjust the current to 3mA, and electrolyze at room temperature for 8h. After the reaction, the reaction liquid was extracted with ethyl acetate, and the corresponding product 3 was obtained after separation and purification, and the yield of the product 3 was 60%.
Embodiment 3
[0037] With metal platinum wire as anode and platinum sheet as cathode, add 0.75mmol sodium fluoroborate, 0.045mmol nickel chloride, 0.3mmol 2,6-dimethylphenol, 0.21mmol trifluoroacetic acid, 0.09mmol fluorine Potassium chloride, 200uL tert-butanol, 5mL acetonitrile, magnetic stirrer, cover the lid, turn on the power, adjust the current to 4mA, and electrolyze at room temperature for 6.5h. After the reaction, the reaction liquid was extracted with ethyl acetate, and the corresponding product 3 was obtained after separation and purification, and the yield of the product 3 was 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com