Method for leaching metals in anode material of waste lithium ion battery
A lithium-ion battery and positive electrode material technology, which is applied in the field of comprehensive recycling of waste lithium-ion batteries, can solve problems such as low alloy purity, low metal leaching rate, and high impurity content, and achieve high metal selectivity, high metal leaching rate, and The effect of low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
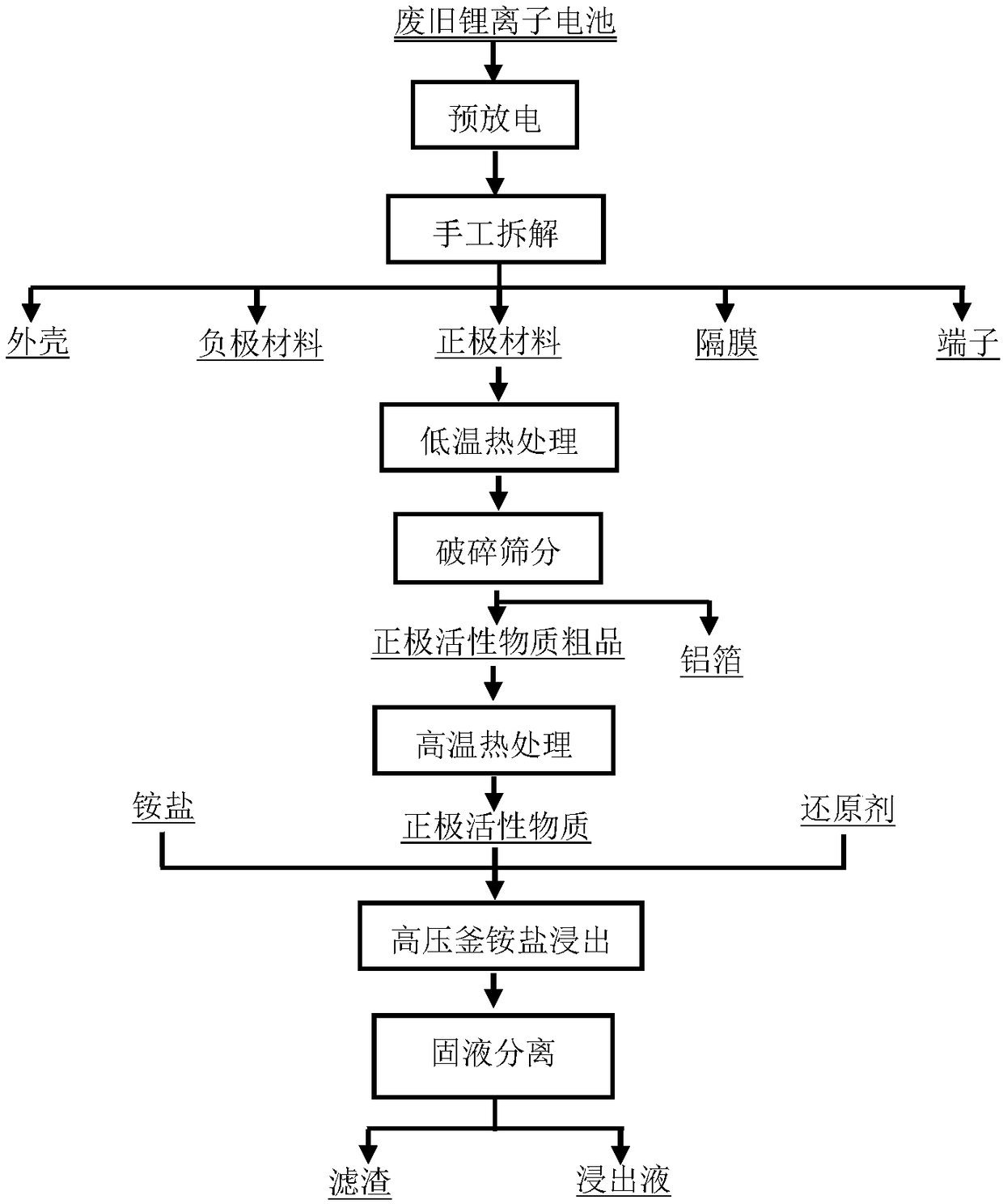
[0043] This embodiment provides a method for leaching the metal in the positive electrode material of the waste lithium-ion battery, and its process flow chart is as follows figure 1 shown, including the following steps:
[0044] 1) Discharge the waste lithium-ion battery by immersing it in a sodium chloride solution with a concentration of 15g / L for 36 hours. After the discharge, disassemble it manually to obtain the positive electrode material, shell, negative electrode material, diaphragm, and terminal;
[0045]2) Put the positive electrode material obtained in step 1) into a muffle furnace and bake at 350° C. for 2 hours;
[0046] 3) The product obtained in step 2) is crushed with a high-speed universal pulverizer, and then sieved with an 80-mesh sieve to obtain the crude positive active material and aluminum foil;
[0047] 4) Put the crude positive active material obtained in step 3) into a muffle furnace for calcination at a high temperature of 650° C. for 2 hours to ob...
Embodiment 2
[0055] This embodiment provides a method for leaching the metal in the positive electrode material of the waste lithium-ion battery, comprising the following steps:
[0056] 1) Soak the waste lithium-ion battery in a sodium chloride solution with a concentration of 15g / L for 36 hours, discharge it, and disassemble it manually after the discharge to obtain the positive electrode material;
[0057] 2) Put the positive electrode material obtained in step 1) into a muffle furnace and bake at 350° C. for 2 hours;
[0058] 3) The product obtained in step 2) is crushed with a high-speed universal pulverizer, and then sieved with an 80-mesh sieve to obtain the crude positive active material and aluminum foil;
[0059] 4) putting the crude positive active material obtained in step 3) into a muffle furnace for calcination at a high temperature of 650° C. for 2 hours to obtain the positive active material;
[0060] 5) Weigh 80g of the positive electrode active material obtained in step ...
Embodiment 3
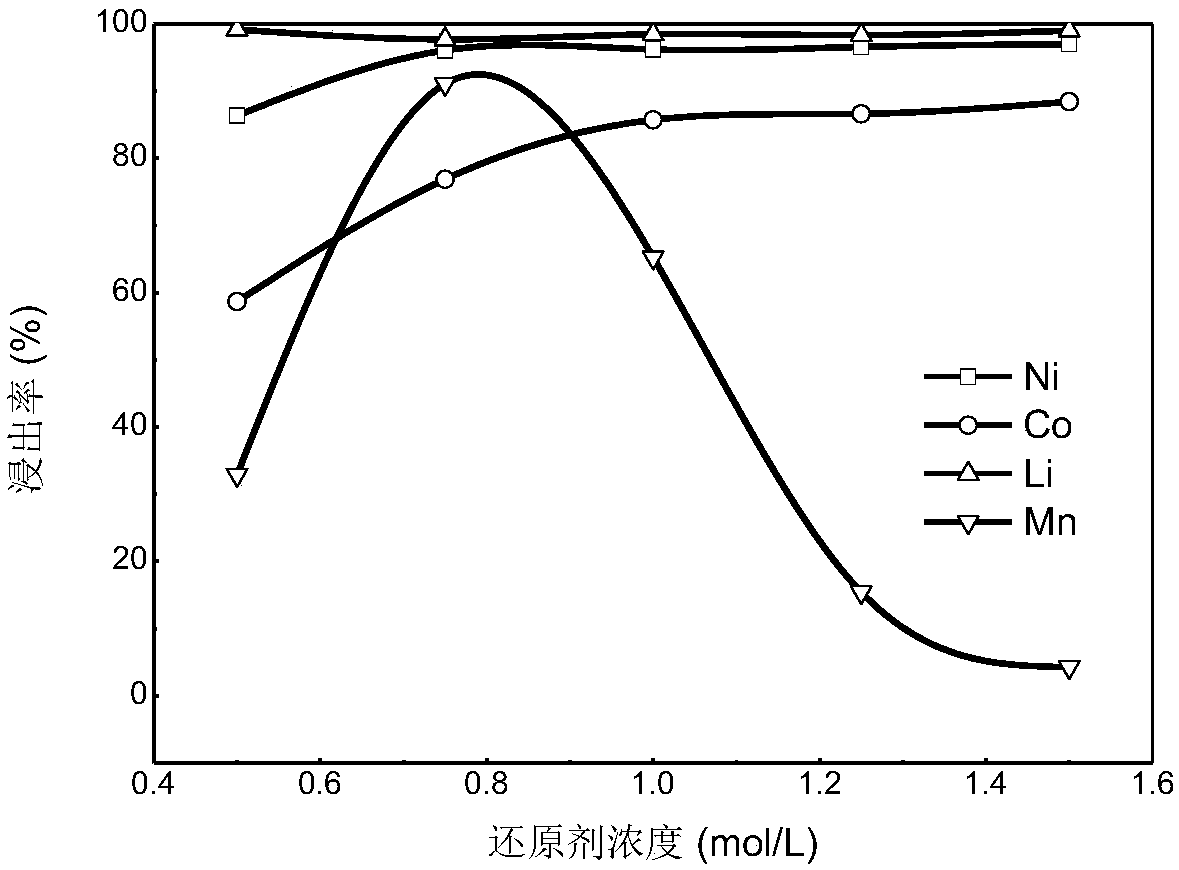
[0066] Be 600mL concentration in the present embodiment and be the ammonium sulfate solution of 5mol / L, all the other are identical with embodiment 1. The leaching rates of various metals in the final leach solution were detected and calculated, and the results showed that the leaching rates of Ni, Co, Mn and Li were 86.64%, 65.31%, 85.02% and 91.01%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com