Preparation method of polysubstituted azatricycloazine derivative
An azatricyclic azine and multi-substitution technology is applied in the field of preparation of multi-substituted azatricyclic azine derivatives, and can solve the problems of incompatibility with green chemistry, poor substrate applicability, complex starting materials and the like, and achieves The effect of post-processing green, short reaction time and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
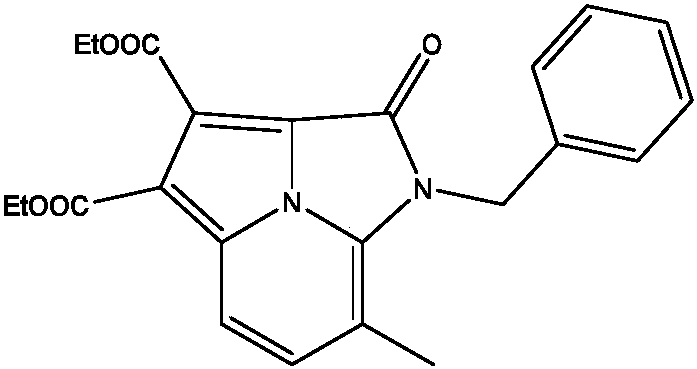
[0022] Preparation of ethyl 1-benzyl-7-methyl-2-carbonyl-1,2-dihydroimidazo[5,1,2-cd]indazine-3,4-dibutyrate
[0023]
[0024] 0.1mmol of N-benzyl-3-methyl-2-aminopyridine, 0.5mmol of dibutyne diethyl ester, 0.002mmol of manganese (III) acetate dihydrate, 0.002mmol of 2,2'-bipyridine, di Add 0.2 mmol of tert-butyl peroxide to an open reaction tube, add 2 mL of acetonitrile, place in an oil bath at 70° C., and react for 16 h. Remove the heat source from the reaction and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 34.6 mg of the target product with a yield of 85%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ7.85(d, J=8.7Hz, 1H), 7.40(d, J=8.7Hz, 1H), 7.34-7.27(m, 3H), 7.20(d, J=6.9Hz, 2H), 5.46( s, 2H), 4.54(q, J=7.1Hz, 2H), 4.44(q, J=7.1Hz, 2H), 2.44(s, 3H), 1.48(t, J=7.1Hz, 3H), 1.44( t, J=7.1Hz, 3H).13C NMR (100MHz, CDCl 3 )δ 163.2 (double), 155.9, 136.8, 133.0,...
Embodiment 2
[0026] 1-(4-Methylbenzyl)-7-methyl-2-carbonyl-1,2-dihydroimidazo[5,1,2-cd]indazine-3,4-dibutyric acid ethyl ester preparation
[0027]
[0028] 0.1mmol of N-(4-methylbenzyl)-3-methyl-2-aminopyridine, 0.5mmol of dibutyne diethyl ester, 0.002mmol of manganese (III) acetate dihydrate, 2,2'-di Add 0.002 mmol of bipyridine and 0.2 mmol of di-tert-butyl peroxide to an open reaction tube, add 2 mL of acetonitrile, place in an oil bath at 70°C, and react for 16 hours. Remove the heat source from the reaction and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 29.9 mg of the target product with a yield of 71%. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, CDCl 3 )δ7.83(d, J=8.7Hz, 1H), 7.39(d, J=8.7Hz, 1H), 7.13-7.06(m, 4H), 5.41(s, 2H), 4.54(q, J=7.0 Hz, 2H), 4.43(q, J=7.1Hz, 2H), 2.45(s, 3H), 2.30(s, 3H), 1.48(t, J=6.9Hz, 3H), 1.43(t, J=7.0 Hz, 3H). 13 C NMR (100MHz, CDCl 3 )δ 163.2 ...
Embodiment 3
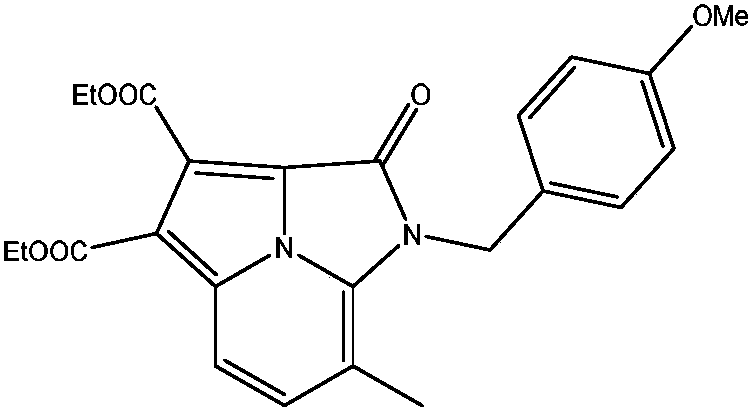
[0030] 1-(4-Methoxybenzyl)-7-methyl-2-carbonyl-1,2-dihydroimidazo[5,1,2-cd]indazine-3,4-dibutyric acid ethyl ester preparation of
[0031]
[0032] 0.1mmol of N-(4-methoxybenzyl)-3-methyl-2-aminopyridine, 0.5mmol of dibutyldiacetylene, 0.002mmol of manganese (III) acetate dihydrate, 2,2'- Add 0.002 mmol of bipyridine and 0.2 mmol of di-tert-butyl peroxide to an open reaction tube, add 2 mL of acetonitrile, place in an oil bath at 70°C, and react for 16 hours. Remove the heat source from the reaction and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 36.2 mg of the target product with a yield of 83%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ7.82(d, J=8.7Hz, 1H), 7.39(d, J=8.7Hz, 1H), 7.14(d, J=8.5Hz, 2H), 6.83(d, J=8.6Hz, 2H) , 5.38(s, 2H), 4.54(q, J=7.1Hz, 2H), 4.43(q, J=7.1Hz, 2H), 3.76(s, 3H), 2.47(s, 3H), 1.48(t, J=7.1Hz, 3H), 1.43(t, J=7.1Hz, 3H). 13 C NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com