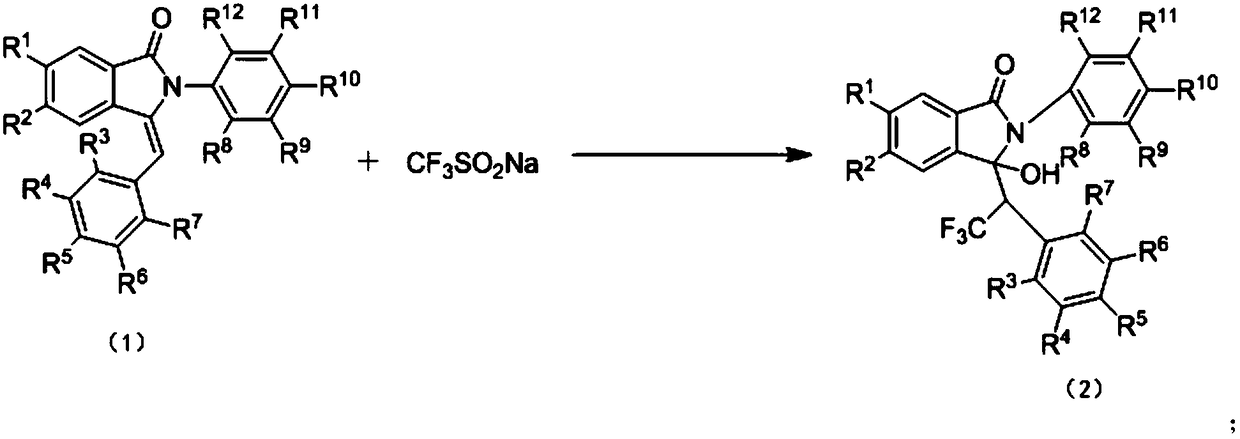
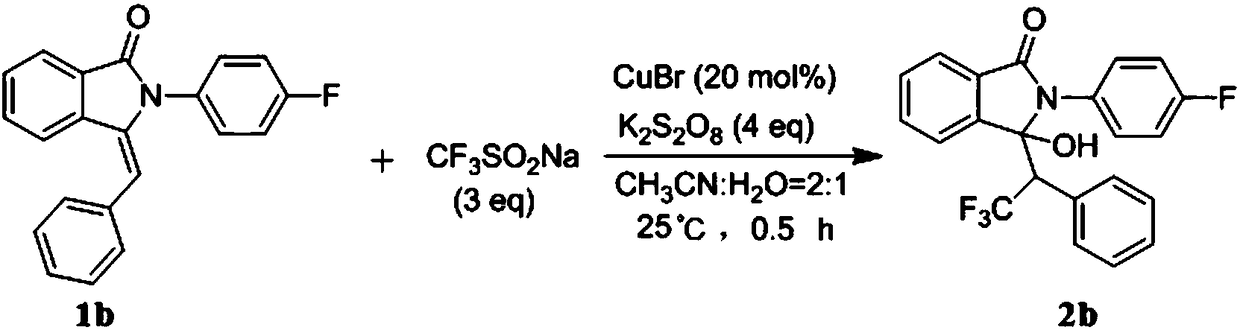Preparation method of trifluoromethyl hydroxylated derivatives of isoindolinone
A technology of trifluoromethyl hydroxy and isoindolinone, applied in the direction of organic chemistry and the like, can solve the problems of complex reaction conditions, poor reaction selectivity, cumbersome steps, etc., and achieves simple reaction operation and post-treatment process, mild reaction conditions, The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
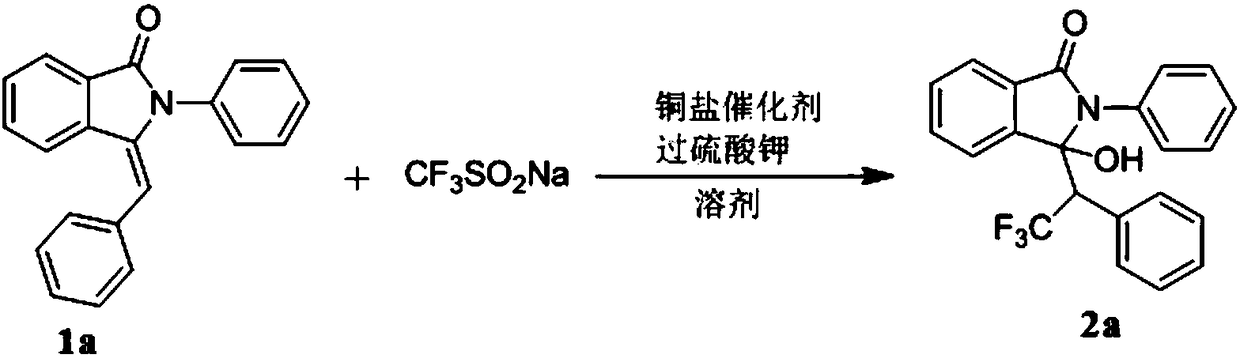
[0037] Embodiment one: the synthesis (2a) of 3-hydroxyl-2-phenyl-3-(2,2,2-trifluoro-1-phenylethyl) isoindol-1-one
[0038]
[0039] (1) Weigh 3-benzylidene-2-phenylisoindol-1-one 1a (0.297g, 1mmol), sodium trifluoromethanesulfinate (0.468g, 3mmol), cuprous chloride (0.020g, 0.2mmol), potassium persulfate (1.081g, 4mmol) was dissolved in 9mL of acetonitrile aqueous solution (acetonitrile: water = 2:1). The mixture was stirred and reacted at 25°C, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:6) to obtain compound 2a. Isolated yield was 60%.
[0040] (2) Weigh 3-benzylidene-2-phenylisoindol-1-one 1a (0.297g, 1mmol), sodium trifluoromethanesulfinate (0.468g, 3mmol), cuprous iodide (0.038g, 0.2mmol), potassium persulfate (1.081g, 4mmol) was dissolved in 9mL of acetonitrile aqueous solution (acetonitrile:water=2:1). Th...
Embodiment 2
[0057] Embodiment two: the synthesis of 3-hydroxyl-2-(4-fluorophenyl)-3-(2,2,2-trifluoro-1-phenylethyl)isoindol-1-one (2b) synthesis
[0058]
[0059] Weigh 3-benzylidene-2-(4-fluorophenyl)isoindol-1-one 1b (0.316g, 1mmol), sodium trifluoromethanesulfinate (0.468g, 3mmol), bromide Cuprous (0.028g, 0.2mmol), potassium persulfate (1.081g, 4mmol) were dissolved in 9mL of acetonitrile aqueous solution (acetonitrile:water=2:1). The mixture was stirred and reacted at 25°C, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:6) to obtain compound 2b. Isolated yield was 67%.
[0060] 2b: Pale yellow solid, 67% yield; 1 H NMR (400MHz, DMSO-d6) δ: 8.00 (s, 1H), 7.93 (d, J = 7.5Hz, 1H), 7.87–7.83 (m, 1H), 7.72 (d, J = 6.8Hz, 1H) ,7.68(d,J=7.2Hz,1H),7.37(dd,J=8.6,5.1Hz,2H),7.29(d,J=9.8Hz,1H),7.26–7.22(m,2H),7.07( t, J=7.6Hz, 2H),...
Embodiment 3
[0061] Example Three: 3-Hydroxy-2-(4-methoxyphenyl)-3-(2,2,2-trifluoro-1-phenylethyl)isoindol-1-one (2c) synthesis
[0062]
[0063] Weigh 3-benzylidene-2-(4-methoxyphenyl)isoindol-1-one 1c (0.327g, 1mmol), sodium trifluoromethylsulfinate (0.468g, 3mmol), Cuprous bromide (0.028g, 0.2mmol), potassium persulfate (1.081g, 4mmol) were dissolved in 9mL of acetonitrile aqueous solution (acetonitrile:water=2:1). The mixture was stirred and reacted at 25°C, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:6) to obtain compound 2c. Isolated yield was 78%.
[0064] 2c: Pale yellow solid, 78% yield; 1 H NMR (400MHz, DMSO-d6) δ: 7.89(d, J=7.4Hz, 1H), 7.85(s, 1H), 7.69–7.63(m, 2H), 7.24(dd, 4H), 7.07(t, J=7.7Hz, 2H), 6.98(d, J=9.0Hz, 2H), 6.46(d, J=7.5Hz, 2H), 4.05(q, J=10.5Hz, 1H), 3.80(s, 3H) ; 13 C NMR (101MHz, DMSO-d6) δ: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com