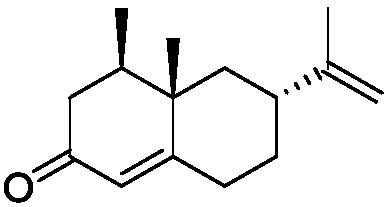
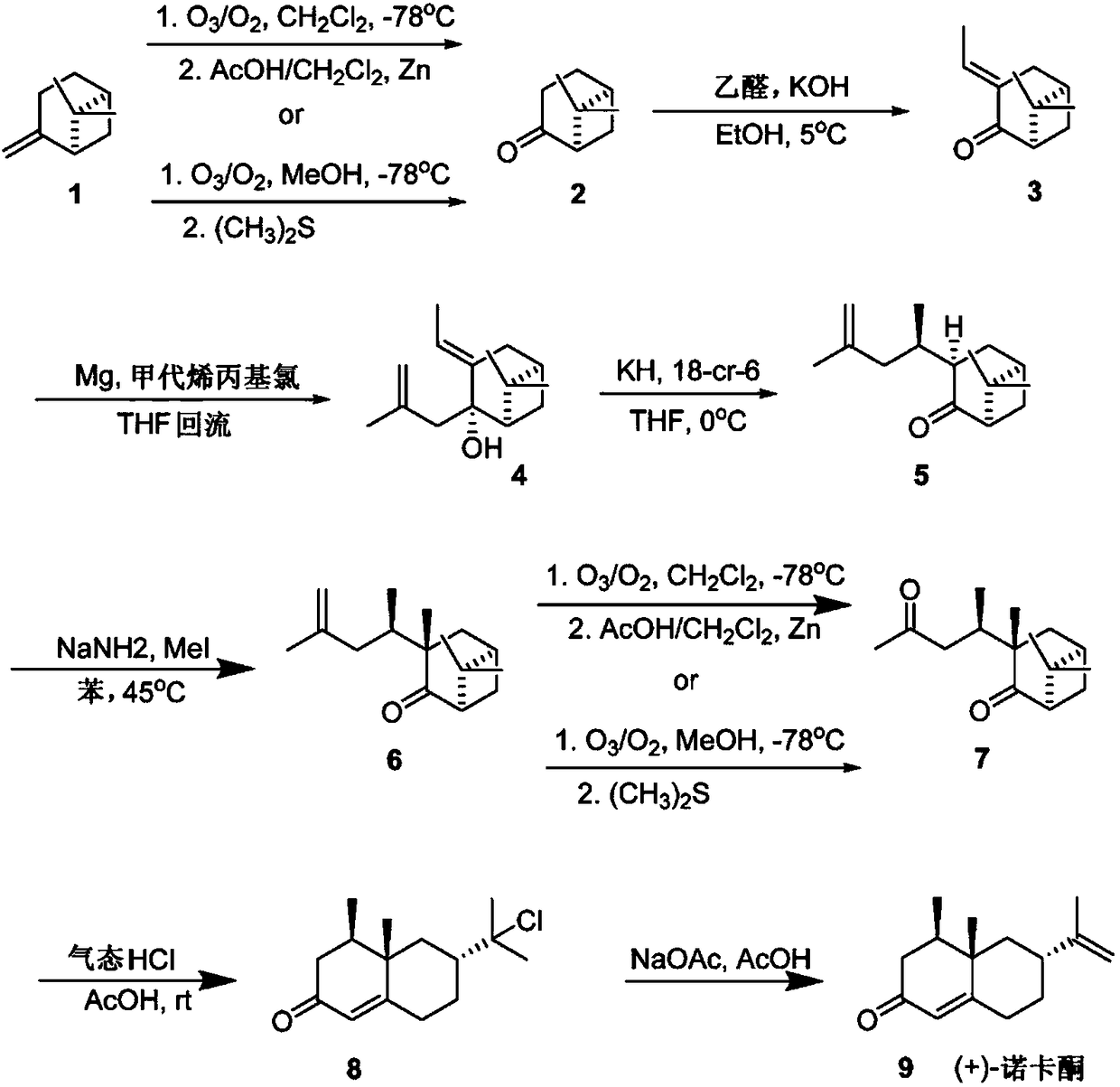
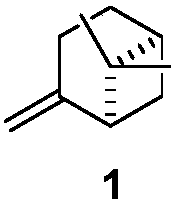
Reaction sequence for the synthesis of nootkatone, dihydronootkatone, and tetrahydronootkatone
A technology of dihydronokadone and tetrahydronokadone, which is applied in the field of reaction sequence for synthesizing nokadone, dihydronokadone and tetrahydronokadone, can solve the problem of low yield and hinder commercial application , expensive raw materials and other issues, to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0018] 6,6-Dimethyl-bicyclo[3.3.1]heptan-2-one, nopinone (compound 2): freeze in 160 ml of CH on dry ice at -78 °C 2 Cl 2 β-pinene (compound 1) (40.0g, 294mmol) solution in, and by ozone / O 2 Bubble overnight until blue color persists. Nitrogen was then bubbled through the reaction mixture for 30 minutes. Add CH 2 Cl 2 (160ml) followed by the addition of AcOH (80ml). At 0°C, Zn (40 g) was added in 1 g portions over 4 hours, dry ice was added to the solution to control the temperature. The solution was rotated on a rotary evaporator at 30 °C for 1 h. TLC (10% EtOAc / Hexane, 5% H 2 SO 4 / EtOH dye) showed complete reaction. Then 300ml of water were added and stirred for 10 minutes. Zn was removed by filtration and CH 2 Cl 2 rinse. CH 2 Cl 2 layer and washed again with water, followed by saturated Na 2 CO 3 solution, followed by brine, MgSO 4 Drying and concentration afforded 33.1 g (82% yield) of a colorless oil. The crude product was used directly in the next st...
Embodiment 1B
[0020] 6,6-Dimethyl-bicyclo[3.3.1]heptan-2-one, nopinone (compound 2): Ozone was bubbled through β- Pinene (compound 1) 4.60mL (29.36mmol, 1 equivalent) solution. After 3 hours, use O again 2 Bubbling was carried out for 30 minutes, after which a characteristic blue color in the solution indicated excess ozone and substrate depletion. Adding (CH 3 ) 2 After S (3.7 mL, 49.91 mmol, 1.7 equiv), the mixture was allowed to warm to room temperature and stirred for another 12 h. Transfer the reaction mixture to a separatory funnel and use CH 2 Cl 2 (10mL) and H 2 O (10 mL) diluted. Collect the organic phase and use CH 2 Cl 2 (2 x 10 mL) to extract the aqueous layer. The combined organic extracts were washed with brine and washed with Na 2 SO 4 dry. Concentration under reduced pressure yielded an oil. Flash column chromatography on silica gel using 3.5% to 8.5% EtOAc in hexanes as the detergent yielded a clear oil which was confirmed to be the title compound by NMR spect...
Embodiment 2
[0022] (1R,5R)-6,6-Dimethyl-3-E-ethylenebicyclo[3.3.1]heptan-2-one (Compound 3): Nopinone (9.9g, 71.6mmol) The solution with EtOH (172ml) was cooled to 5°C and KOH (4.72g) was added while stirring. Acetaldehyde (5.74ml dissolved in 43ml EtOH) was added to the reaction over 30 minutes. Four additional portions of acetaldehyde (same amount) were added to the solution at 12 h intervals. The reaction was stirred for an additional 6 hours. p-Toluenesulfonic acid monohydrate was added and the solution was concentrated on a rotary evaporator. Add a small amount of CH 2 Cl 2to dissolve the residue, then hexane was added and the solution was vortexed. The solution was transferred to another flask and concentrated. After flash chromatography (10% EtOAc in hexanes), a colorless oil was obtained (8.6 g, 73% yield). Further purification using Kugelrohr distillation (250°C, 10 mmHg) afforded 6.5 g of product (55% yield). 1 HNMR: (250MHz, CDCl 3 ), δ6.89–6.86(m,1H),2.59–2.56(m,4H),2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com