Meldrum's acid, barbituric acid and pyrazolone derivatives substituted with hydroxylamine as hno donors
A technology of substituents and compounds, applied in the field of hydroxylamine-substituted Michaelis acid, barbituric acid and pyrazolone derivatives as HNO donors, can solve the problems of less HNO donors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0160] The following examples are presented for illustrative purposes and should not be used to limit the scope of the disclosed subject matter.
example 1
[0161] Example 1: Synthetic Compounds
[0162] Compounds described herein can be prepared according to the general methods described in Schemes 1 to 3 or by procedures known in the art. Starting materials for the reactions are either commercially available or may be prepared by known procedures or obvious modifications thereof. For example, 5-bromo-5-methyl Michaelis acid is obtained by bromination of 5-methyl-Miners acid (sodium bicarbonate, bromine, water) 1 . 5-Acetyl-Milleis acid was obtained by acylation of Michaelis acid (acetic acid, N,N'-dicyclohexylcarbodiimide, 4-dimethylaminopyridine, dichloromethane) 2 . 5-Acetyl-N,N-dimethylbarbituric acid obtained by acylation of N,N-dimethylbarbituric acid (acetyl chloride, pyridine, dichloromethane) 2,3 . 5-Ethyl-barbituric acid is obtained by reduction of 5-acetyl-Meeter's acid (sodium cyanoborohydride, acetic acid) 4 . 4-Acetyl-N-phenyl-5-methyl-pyrazolone obtained by acylation of N-phenyl-5-methyl-pyrazolone (acetyl c...
example 2
[0209] Example 2: HNO production
[0210] The compounds described herein are believed to donate HNO based on the general strategy shown in Scheme 4, where X is a leaving group. Piloty's acid and its derivatives with a sulfinate leaving group are typical examples of this strategy. The compounds described herein employ a carbon-based leaving group to allow the release of HNO at neutral pH along with a stabilizing carbanion.
[0211] Process 4. HNO release
[0212] General process:
[0213]
[0214] specific instance:
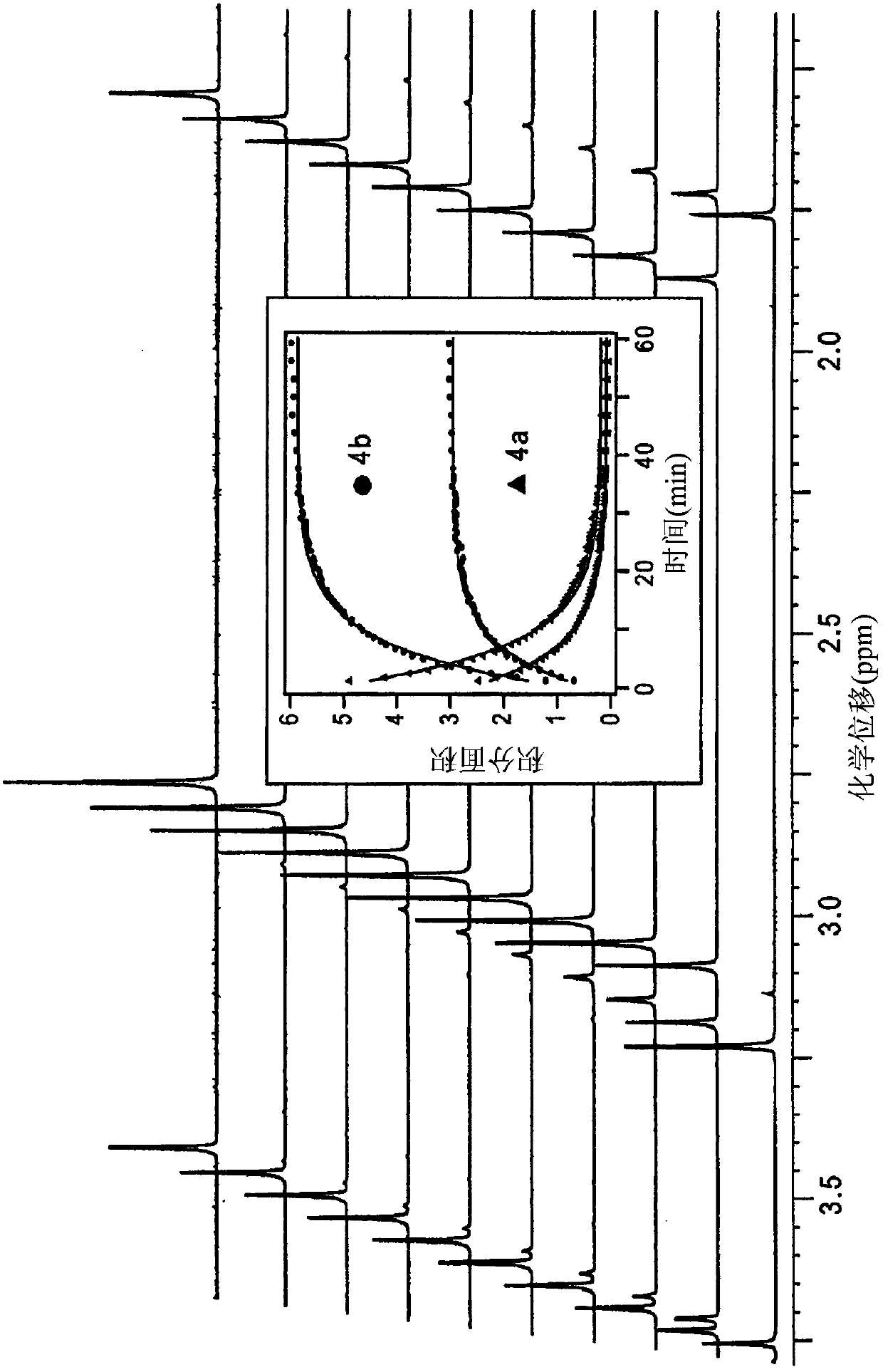
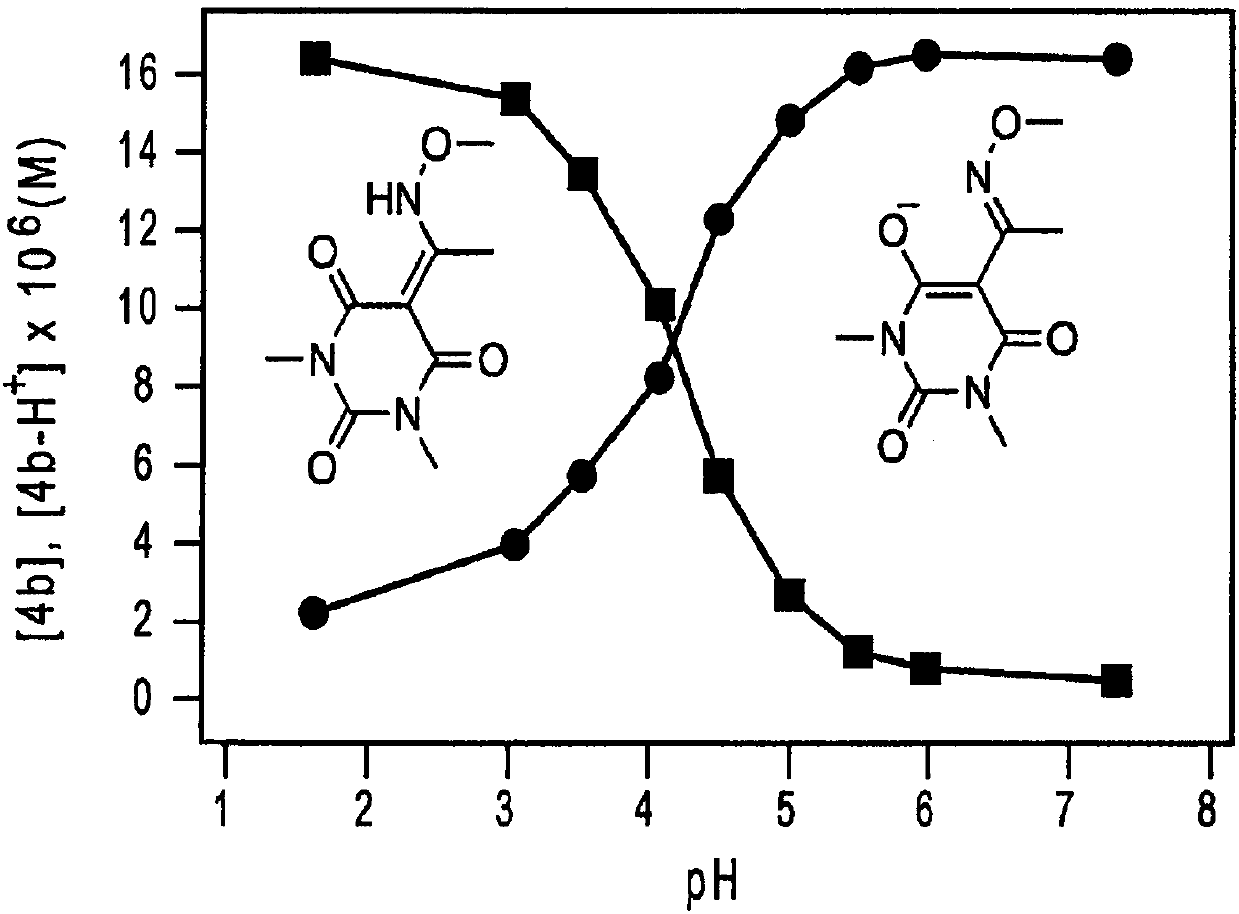
[0215] (a) HNO release from Michaelis acid derivatives 1a and 2a at pH 7.4 at 37°C
[0216]
[0217] (b) HNO release from barbituric acid 3a and 4a at pH 7.4 at 37 °C
[0218]
[0219] (c) HNO release from pyrazolones 6a-12a at pH 7.4 at 37 °C
[0220]
[0221] Nitrous oxide is prepared by dimerization and dehydration of HNO and is the most common marker of HNO production (Fukuto, J.M. et al., Chem. Res. Toxicol. 2005, 18, 790-801 ). However, HNO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com