Escherichia coli CG1061 for effectively degrading aflatoxin B1
An aflatoxin and Escherichia coli technology, applied in bacteria, microorganism-based methods, microorganisms, etc., can solve the problems of unclear toxicity, limited types, and application of degrading bacteria metabolites, and achieve good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
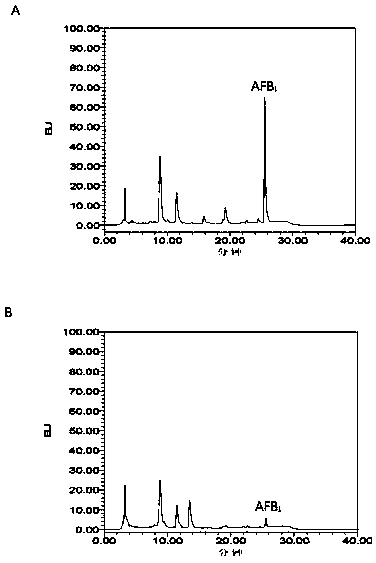
[0025] Example 1 Chicken cecum microorganism AFB 1 Degradative activity
[0026] 1. Method
[0027] (1) Randomly buy a live chicken from Wushan Vegetable Market in Guangzhou. After slaughtering, take out the cecum, cut open the cecum to get its contents, add 10ml of LB medium, mix well, let it stand for 10min, and the supernatant is the cecal mixed microorganisms.
[0028] (2) Add 100μl to 900μl LB medium, add 250μg / ml AFB 1 10 μl of the mother solution to make the final concentration 2.5 μg / ml.
[0029] (3) Culture at 37°C, 180r / min, and dark for 3 days.
[0030] (4) Take 200μl and add the same amount of dichloromethane to extract three times, centrifuge at 10000rpm for 1min, and wash the supernatant with N 2 blow dry. Add 150 μL of methanol to fully dissolve the residue, and filter it to the sample bottle with a 0.22 μm nylon needle filter.
[0031] (5) Use high performance liquid chromatography (HPLC) for AFB 1 Residues are tested.
[0032] (6) AFB 1 Metabolic rate...
Embodiment 2
[0034] Example 2 Isolation and identification of Escherichia coli CG1061
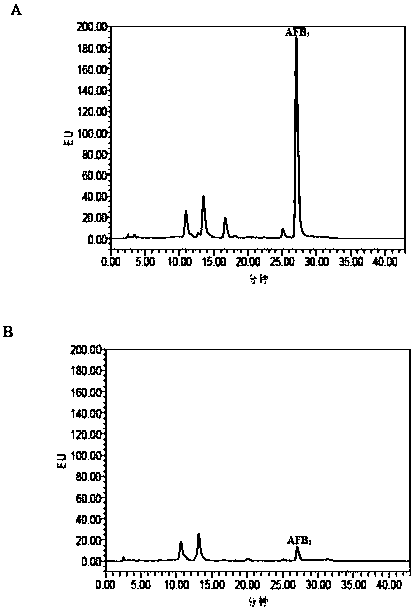
[0035] 1. Screening of AFB by plate separation method 1 Degrading bacteria
[0036] Carry out the dilution plate method separation AFB to the chicken gut microbiota described in embodiment 1 1 degrading bacteria. Pour the sterilized LB solid medium into a sterile plate while it is hot, and set it aside after solidification. Chicken intestinal microbial flora was serially diluted, and 10 -5 、10 -6 Draw 200 μL of the bacterial suspension from the two bacterial dilutions and put it in the center of the plate, do 3 repetitions for each dilution, spread evenly on the surface of the medium with a sterile glass coating stick, and let it stand at room temperature for 5 ~10min. All the plates were placed upside down in a constant temperature incubator at 37°C for 24 hours. According to the shape of the colonies, 50 single colonies were randomly picked, respectively inserted into 96-well cell culture plate...
Embodiment 3
[0040] Example 3 Escherichia coli CG1061 pathogenicity detection
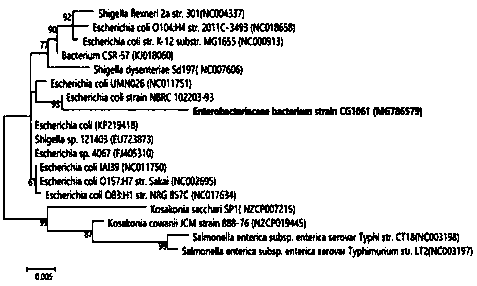
[0041] 1. Extract Escherichia coli CG1061 genomic DNA according to the instructions of the DNA extraction kit, and perform multiplex PCR according to the instructions of the DiarrhoeogenicE. coli PCR Kit (Statens Serum Institut, Denmark) to detect the ten pathogenic genes currently reported ipa H, aat A, elt A, vtx 2, eae , agg R, vtx 1, aai C, est A-porcine, est The expression of A-human.
[0042] 2. The pathogenicity test results of Escherichia coli CG1061 are attached Figure 4 shown. The results showed that Escherichia coli CG1061 did not carry the above-mentioned known pathogenic genes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com