Gelatin microsphere/magnesium phosphate-based bone cement medicine slow-release carrier and preparation method thereof
A magnesium phosphate-based bone, slow-release carrier technology, used in pharmaceutical formulations, drug delivery, prostheses, etc., can solve the problems of insignificant long-term sustained-release effect, fast drug release, and inability to achieve sustained-release, to promote potential Ability, promote bone repair, maintain activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
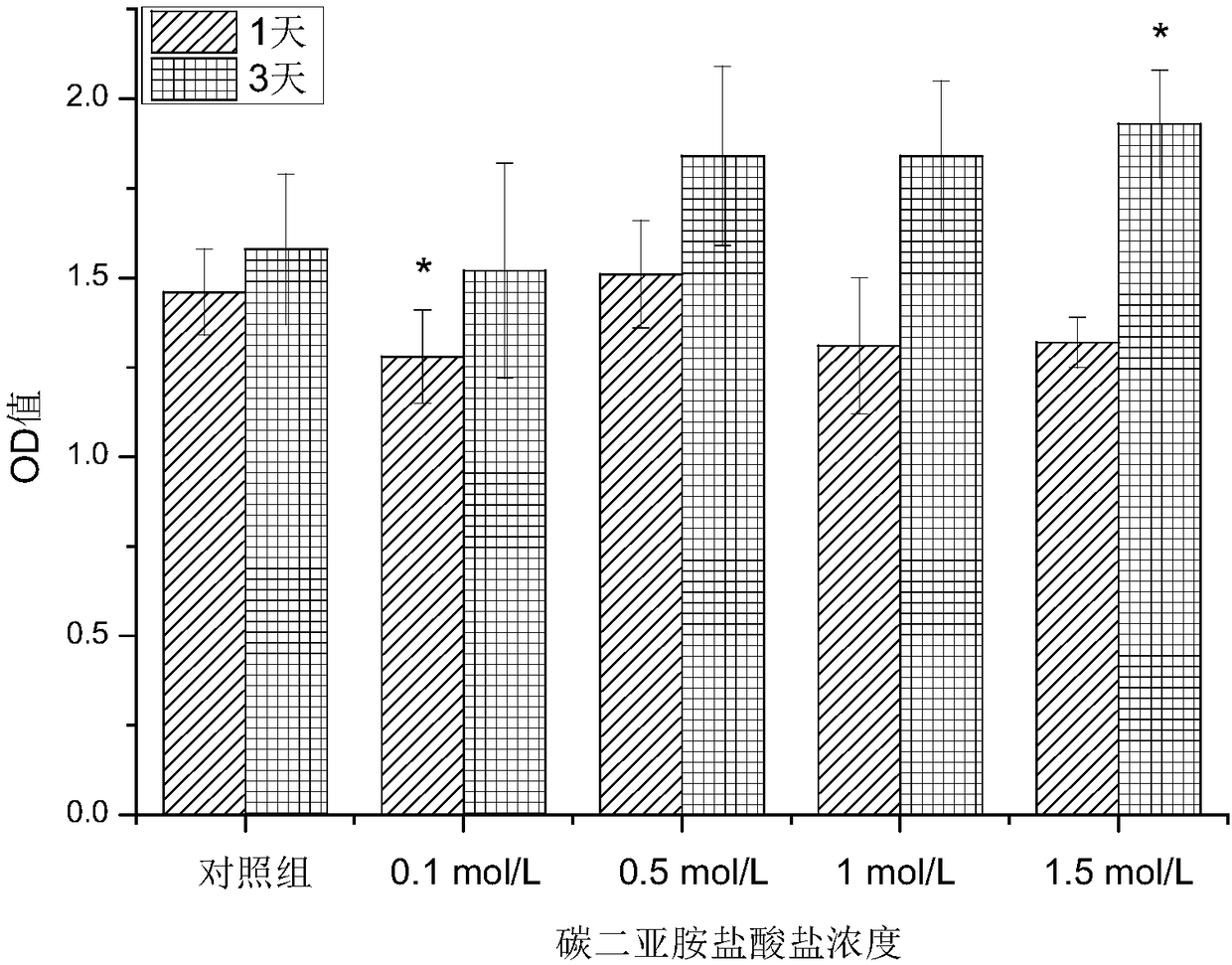
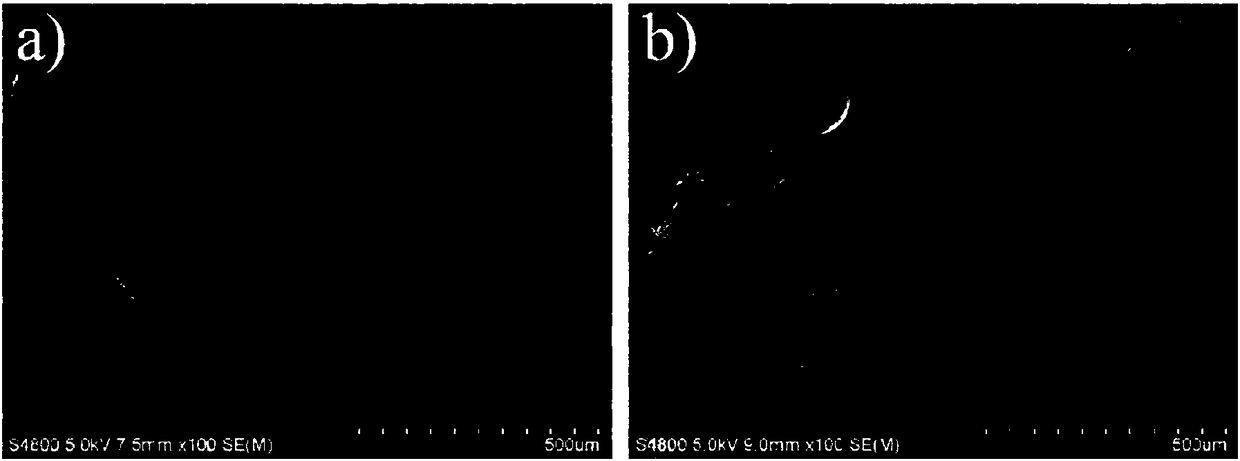
[0034] Weigh 1.5 g of gelatin microspheres and 0.03 g of diclofenac sodium and dissolve them in distilled water at 50° C. to prepare a uniformly mixed pharmaceutical gelatin aqueous solution. Take another 30mL of liquid paraffin and 3mL of Span 80 in a round bottom flask, stir for 10min at a speed of 500r / min and a temperature of 50°C, then slowly add the drug gelatin solution dropwise into the flask at a speed of 250r / min, and stir After 10 minutes, quickly use an ice-water bath, and after 15 minutes, add 1 mL of carbodiimide hydrochloride solution with a concentration of 0.5 mol / L. Continue stirring and cross-linking for 60 minutes, take it out, wash with isopropanol, and dry at room temperature to obtain gelatin microspheres loaded with diclofenac sodium. The morphology of the microspheres was observed with a scanning electron microscope, such as figure 2 shown.
[0035]The bone cement powder is prepared by uniformly mixing magnesium oxide, potassium dihydrogen phosphate...
Embodiment 2
[0038] Weigh 1.5 g of gelatin microspheres and 0.03 g of diclofenac sodium and dissolve them in distilled water at 50° C. to prepare a uniformly mixed pharmaceutical gelatin aqueous solution. Take another 30mL of liquid paraffin and 3mL of Span 80 in a round bottom flask, stir for 10min at a speed of 500r / min and a temperature of 50°C, then slowly add the drug gelatin solution dropwise into the flask at a speed of 350r / min, and stir After 10 minutes, quickly use an ice-water bath, and after 15 minutes, add 1 mL of carbodiimide hydrochloride solution with a concentration of 1 mol / L. Continue stirring and cross-linking for 60 minutes, take it out, wash with isopropanol, and dry at room temperature to obtain gelatin microspheres loaded with diclofenac sodium. The part where the gelatin microspheres and bone cement are combined is the same as in Example 1.
[0039] The drug-loaded gelatin microsphere has good sphere-forming effect, and the particle size is 0-250 μm. The strength...
Embodiment 3
[0041] Weigh 1.5 g of gelatin microspheres and 0.03 g of diclofenac sodium and dissolve them in distilled water at 50° C. to prepare a uniformly mixed pharmaceutical gelatin aqueous solution. Take another 30mL of liquid paraffin and 3mL of Span 80 in a round bottom flask, stir for 10min at a speed of 500r / min and a temperature of 50°C, then slowly add the drug gelatin solution dropwise into the flask at a speed of 350r / min, and stir After 10 minutes, quickly use an ice-water bath, and after 15 minutes, add 1 mL of carbodiimide hydrochloride solution with a concentration of 0.1 mol / L. Continue stirring and cross-linking for 60 minutes, take it out, wash with isopropanol, and dry at room temperature to obtain gelatin microspheres loaded with diclofenac sodium. The part where the gelatin microspheres and bone cement are combined is the same as in Example 1.
[0042] The drug-loaded gelatin microsphere has good sphere-forming effect, and the particle size is 0-250 μm. The streng...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com