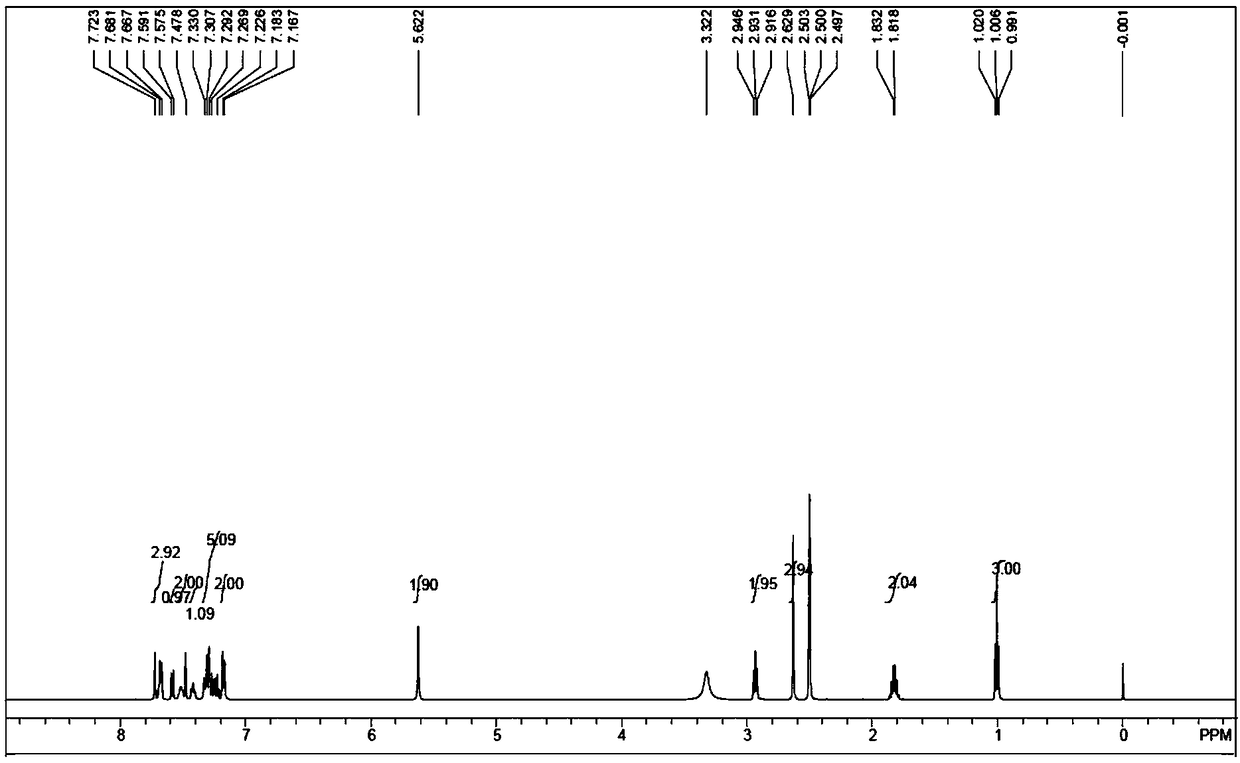
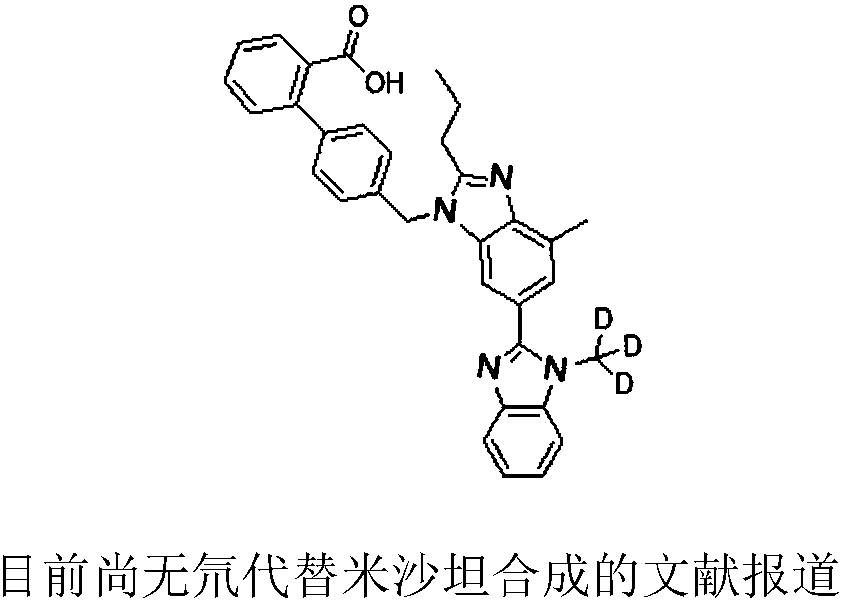
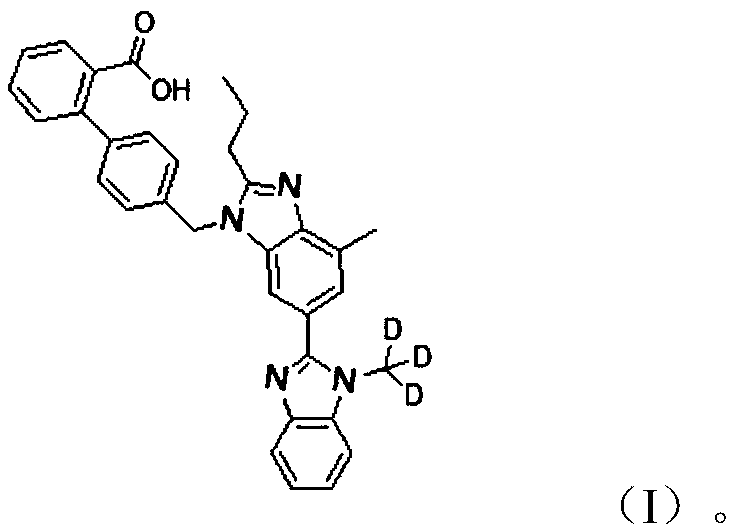
Deuterated methyl telmisartan as well as preparation method and application thereof
A technology of telmisartan and deuterated methyl, applied in the field of deuterated methyl telmisartan and its preparation, can solve the problems of limited use, high price, etc. Well-designed effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] A kind of preparation method that deuterium replaces misartan, it comprises the following steps:
[0053] 1): Dissolving 3-methyl-4-nitrobenzoic acid in ethanol, adding thionyl chloride dropwise, heating under reflux to obtain compound (2);
[0054]
[0055] 2): dissolving compound (2) in a solvent, and hydrogenating palladium carbon to obtain compound (3);
[0056]
[0057] 3): dissolving compound (3) in dichloromethane, adding an alkaline antacid, and adding n-butyryl chloride dropwise at 0°C for acylating reaction to obtain compound (4);
[0058]
[0059] 4): At 0°C, add concentrated sulfuric acid to compound (4), add fuming nitric acid or concentrated nitric acid dropwise, and react to obtain compound (5);
[0060]
[0061] 5): dissolving compound (5) in an acidic solvent, adding a reducing agent, and reducing ring closure to obtain compound (6);
[0062]
[0063] 6): dissolving compound (6) in a solvent, adding a base, and then adding 4-bromobenzyl...
Embodiment 1
[0089] The preparation of compound (2):
[0090]
[0091] Add 3-methyl-4-nitrobenzoic acid (18.1g, 100mmol) into a 500mL dry three-necked flask, add 200mL of absolute ethanol, then ice-bath to 0°C, slowly add 10mL of thionyl chloride dropwise into the three-necked flask, keep the temperature in the three-necked flask not exceeding 10°C, after the dropwise addition, the reaction temperature gradually rises from 0°C to 80°C, and the tail gas is absorbed with aqueous sodium hydroxide solution, and the reaction is completed for 2 hours. Remove methanol and remaining thionyl chloride, obtain white solid 20.1g compound (2),
[0092] yield: 96.1%, LCMS[M+H]=210.
[0093] The preparation of compound (3):
[0094]
[0095] Compound (2) (10.45g, 50mmol) was added to a 150mL single-necked bottle filled with 60mL of EtOH, 1.0g of 10% anhydrous Pd / C was added, and it was reacted overnight at room temperature under a hydrogen atmosphere. TLC monitored that the reaction was basically c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com