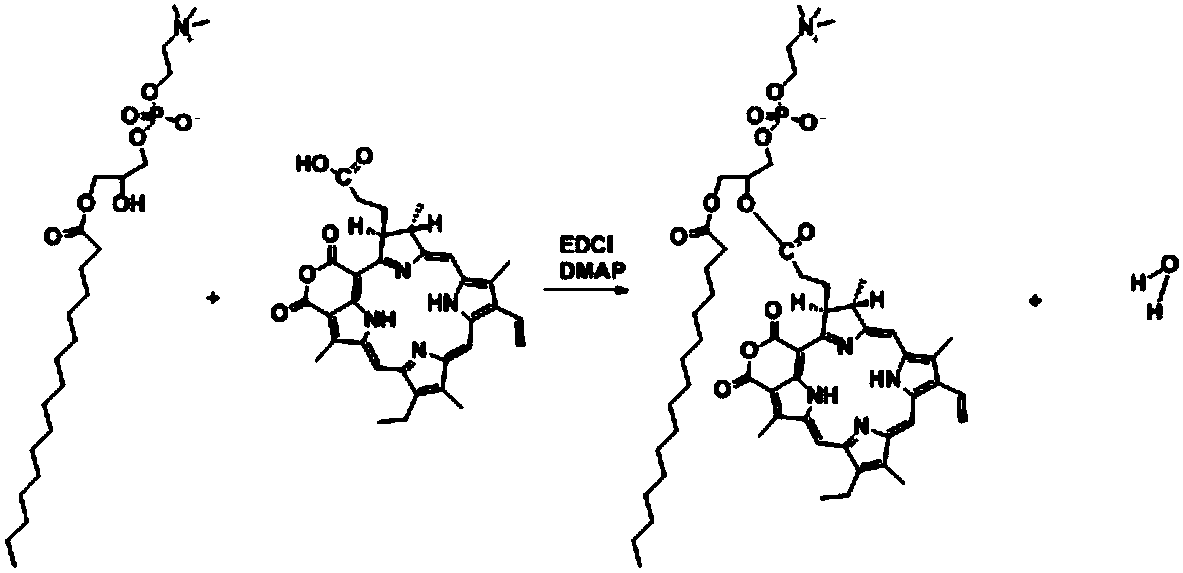
Preparation method of purpurin 18-liposome nanovesicle and application of purpurin 18-liposome nanovesicle to preparation of medicines for treating tumor
A nanovesicle, violetin technology, applied in the directions of antitumor drugs, liposome delivery, drug delivery, etc., can solve the problems of affecting the therapeutic effect of PDT, poor solubility of violetin, and difficulty in uptake, etc., and improve passive targeting performance. , High light-to-heat conversion efficiency, and the effect of increasing the load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
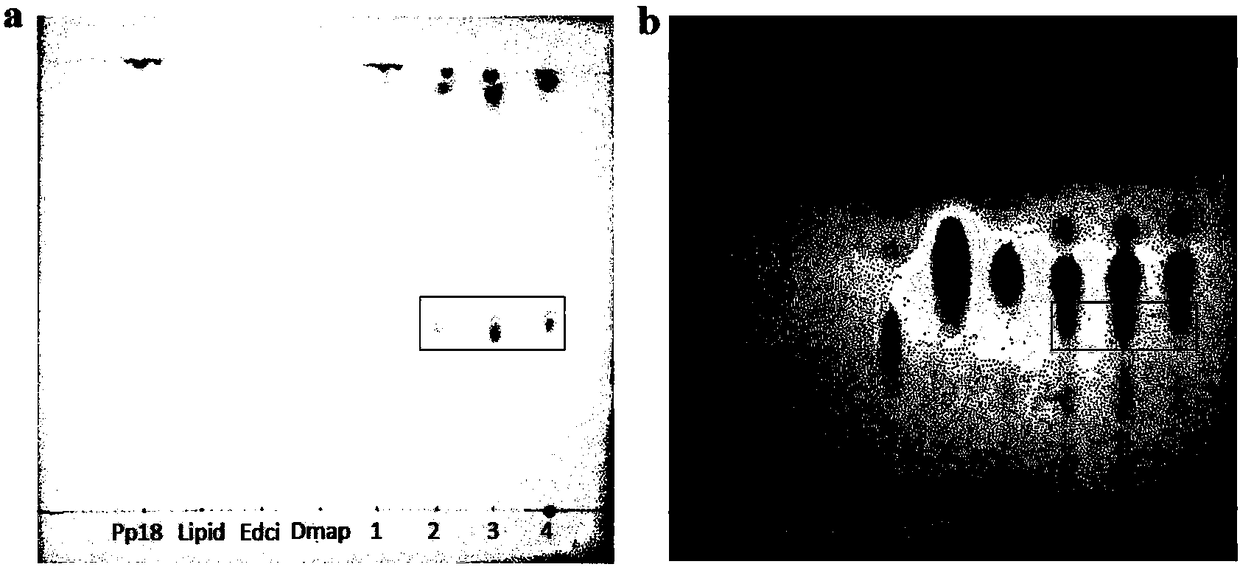
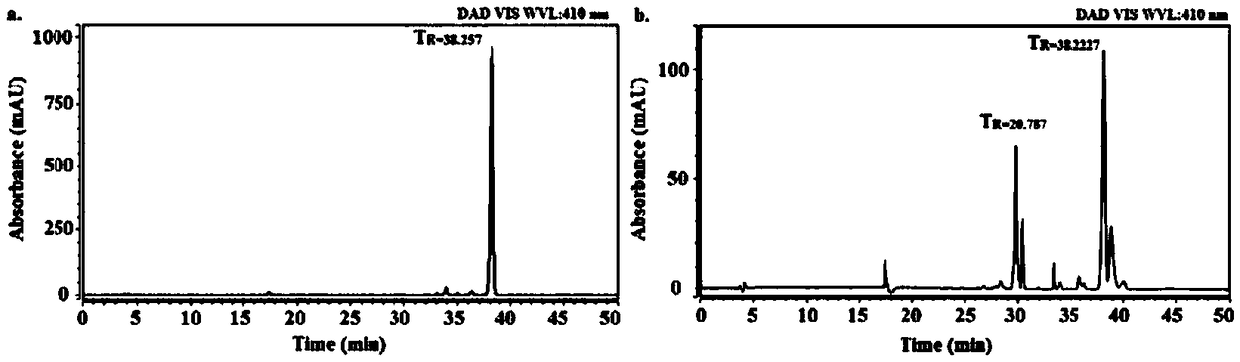
Image
Examples
Embodiment 1
[0068] The synthesis of embodiment 1 purpurin 18-phospholipid
[0069] Weigh Pp18: 56.4mg, P-lyso PC: 98.7mg, EDCI: 38.35mg, DMAP: 24.35mg, dissolve in 10mL of anhydrous chloroform, add in sequence to the reaction bottle filled with argon, and stir at room temperature in the dark After 24 hours, the reaction was completed. Mix the reaction mixture with silica gel powder, evaporate the solvent to dryness at 37°C, and purify using silica gel column chromatography with gradient elution. The ratio is 9:1, ② chloroform: methanol volume ratio is 8.5: 1.5, ③ chloroform: methanol: acetic acid volume ratio is 8: 1.5: 0.5, collect Pp18-lipid, use a rotary evaporator to initially evaporate the solvent, Blow dry with nitrogen, dry in vacuum for 3 h, and store in -20°C refrigerator.
Embodiment 2
[0070] The synthesis of embodiment 2 purpurin 18-phospholipids
[0071] Weigh Pp18: 112.8mg, P-lyso PC: 98.7mg, EDCI: 38.35mg, DMAP: 24.35mg, dissolve in 10mL of anhydrous chloroform, add to the reaction bottle filled with argon in turn, and stir at room temperature in the dark After 24 hours, the reaction was completed. Mix the reaction mixture with silica gel powder, evaporate the solvent to dryness at 37°C, and purify using silica gel column chromatography with gradient elution. The ratio is 9:1, ② chloroform: methanol volume ratio is 8.5: 1.5, ③ chloroform: methanol: acetic acid volume ratio is 8: 1.5: 0.5, collect Pp18-lipid, use a rotary evaporator to initially evaporate the solvent, Blow dry with nitrogen, dry in vacuum for 3 h, and store in -20°C refrigerator.
Embodiment 3
[0072] The synthesis of embodiment 3 purpurin 18-phospholipids
[0073] Weigh Pp18: 112.8mg, P-lyso PC: 98.7mg, EDCI: 76.7mg, DMAP: 48.7mg, dissolve in 10mL of anhydrous chloroform, add in sequence to a reaction bottle filled with argon, and stir at room temperature in the dark After 24 hours, the reaction was completed, the reaction mixture was mixed with silica gel powder, the solvent was evaporated to dryness, and the solvent was purified by silica gel column chromatography, gradient elution, and the polarity of the eluent was in order from high to low. 9:1, ② chloroform: methanol volume ratio is 8.5: 1.5, ③ chloroform: methanol: acetic acid volume ratio is 8: 1.5: 0.5, collect Pp18-lipid, use rotary evaporator to preliminarily evaporate the solvent to dryness, nitrogen blow Dry, vacuum-dry for 3 hours, and store in a -20°C refrigerator.
[0074] By changing the ratio of the reactants Pp18, P-lyso PC and the two catalysts (both EDCI and DMAP are catalysts), when the molar ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com