Neratinib dimaleate crystal forms and preparation method thereof
A technology of maleic acid and crystal form, which is applied in the field of medicine and can solve problems such as inability to maintain stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The preparation method of the crystal form V of the compound of formula (I) comprises the following steps:
[0066] (1) Adding Neratinib free base and maleic acid to the first solvent (an alcoholic organic solvent, preferably methanol) to obtain the first solution;
[0067] (2) drop the second solvent (being an ester organic solvent, preferably isopropyl acetate) into the first solution obtained in step (1), stir (as carried out at room temperature), filter, and vacuum-dry the filter cake, thereby Obtaining the crystal form V of the compound of formula (I);
[0068] The temperature of the vacuum drying is 30-50°C (preferably 40°C).
[0069] In another preferred example, the molar ratio of maleic acid to neratinib free base is 1.8-2.5, preferably 1.9-2.1.
[0070] In another preferred example, the volume ratio of the first solvent to the second solvent is 1:1˜1:3, preferably 1:2.
[0071] The new crystal form of the present invention is the crystal form IV of the comp...
Embodiment 1
[0108] Embodiment 1: Preparation of Form IV
[0109] Weigh 35.2 mg of neratinib free base, add 1 mL of methanol, add 15 mg of maleic acid, dissolve, add dropwise 2 mL of toluene, and evaporate the resulting solution to dryness at 45°C. The resulting solid is Form IV of the compound of formula (I).
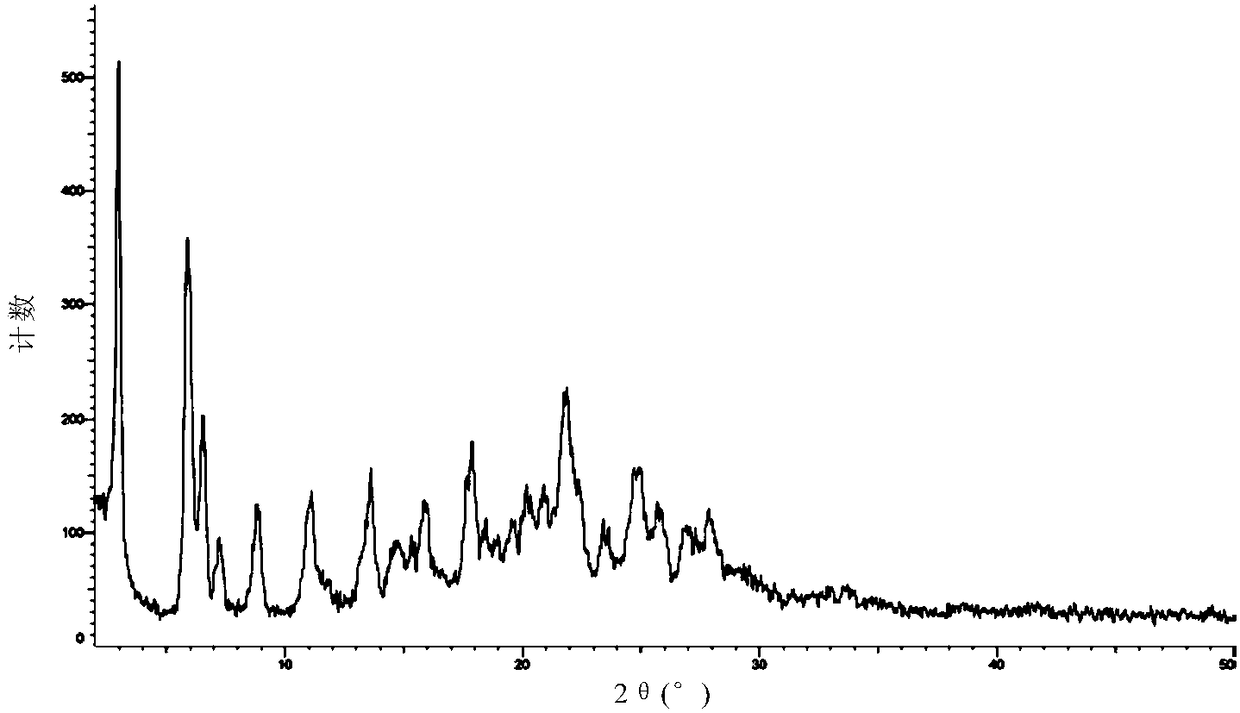
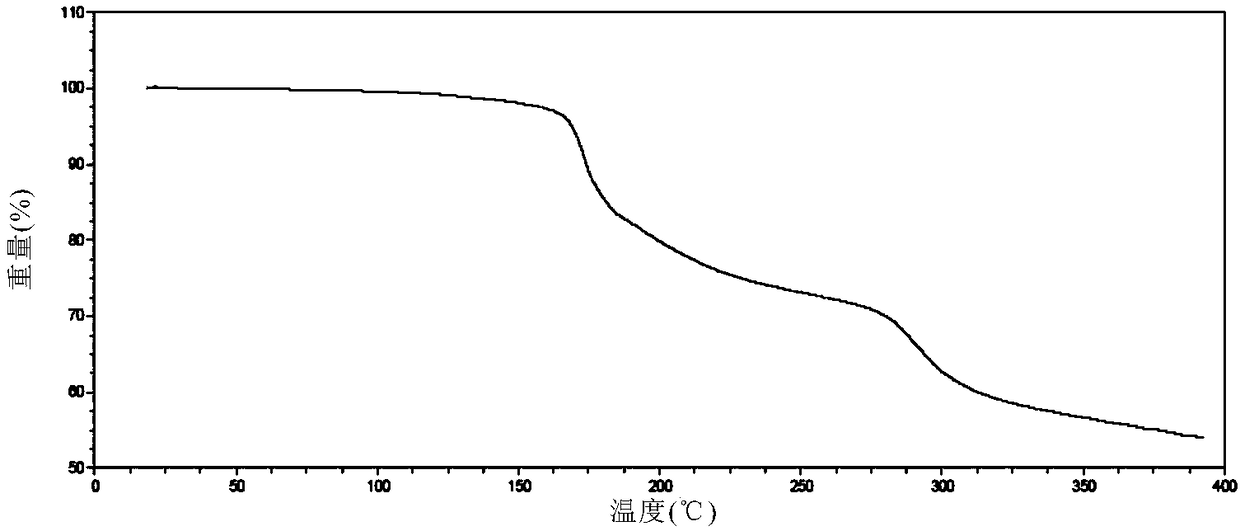
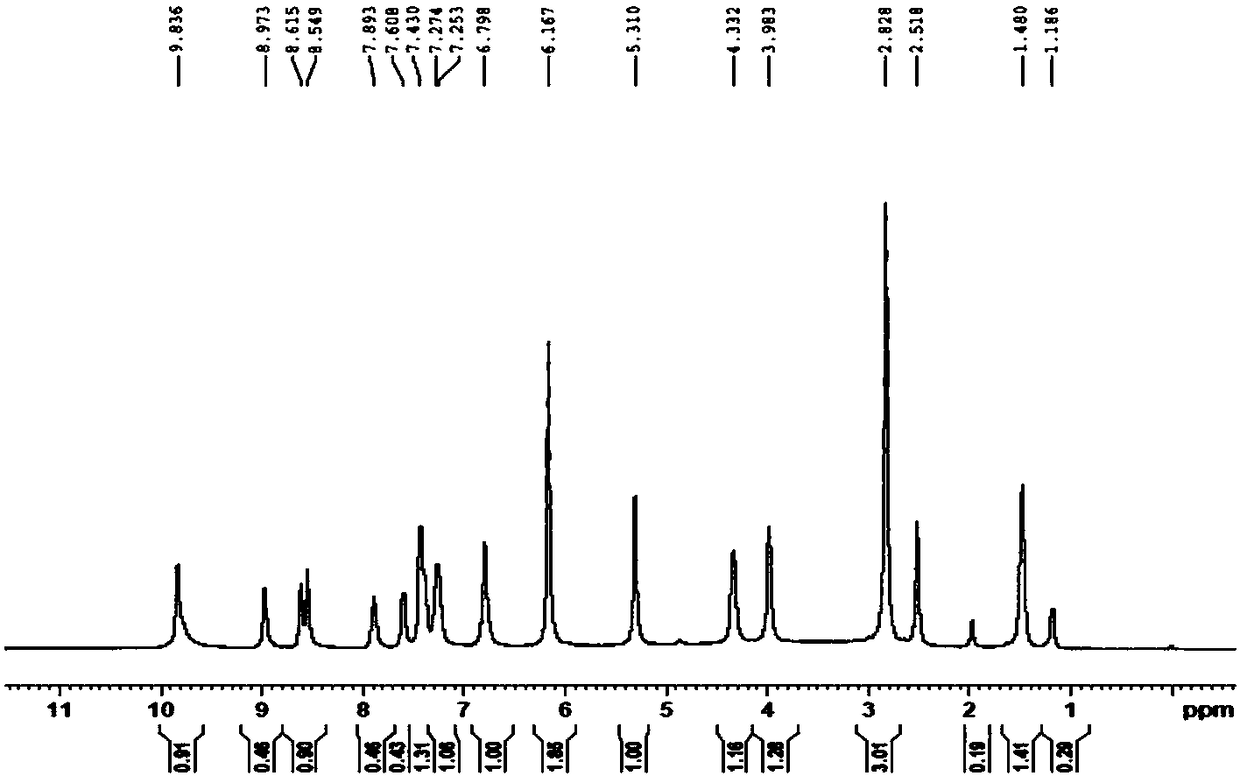
[0110] Carry out XRPD test to the obtained solid, its X-ray powder diffraction data are as shown in table 1, and its X-ray powder diffraction figure is as follows figure 1 shown; the obtained solid was 1 H-NMR test, its spectrum is as follows image 3 Shown, show that the ratio of neratinib and maleic acid is 1:2; Gained solid is carried out TGA test, and its spectrogram is as follows figure 2 Shown, heating to 100 ℃, weight loss 0.8%, no weight loss platform, is anhydrous.
[0111] Table 1
[0112]
[0113]
Embodiment 2
[0114] Embodiment 2: Preparation of Form IV
[0115] Weigh 200mg of neratinib free base, add 5mL of methanol, add 80mg of maleic acid, dissolve, add dropwise 10mL of isopropyl acetate, add a small amount of the crystal form IV seed crystal of the above-mentioned Example 1, and leave it at room temperature overnight, a large amount of solid Precipitated, filtered, and vacuum dried at 25°C to obtain 208.1 mg of sample with a yield of 73.5%. The resulting solid is Form IV of the compound of formula (I).
[0116] Carry out XRPD test to the obtained sample, its X-ray diffraction pattern is basically as figure 1 Shown; Carry out TGA test to the obtained sample, its thermogravimetric analysis diagram is basically as figure 2 shown; the obtained samples were 1 H-NMR test, its 1 The H-NMR spectrum is basically as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com