An industrialized high-density mixed fermentation culture method of bifidobacteria and lactobacillus and a bacteria powder embedding method
A bifidobacterium and lactobacillus technology, applied in the direction of bifidobacteria, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of high cost of culture medium, low survival rate of bacteria powder in vitro, complex industrial production methods, etc. problems, to achieve fast enteric dissolution, improve equipment utilization, and be easy to purchase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, mixed strain high-density fermentation medium screening:
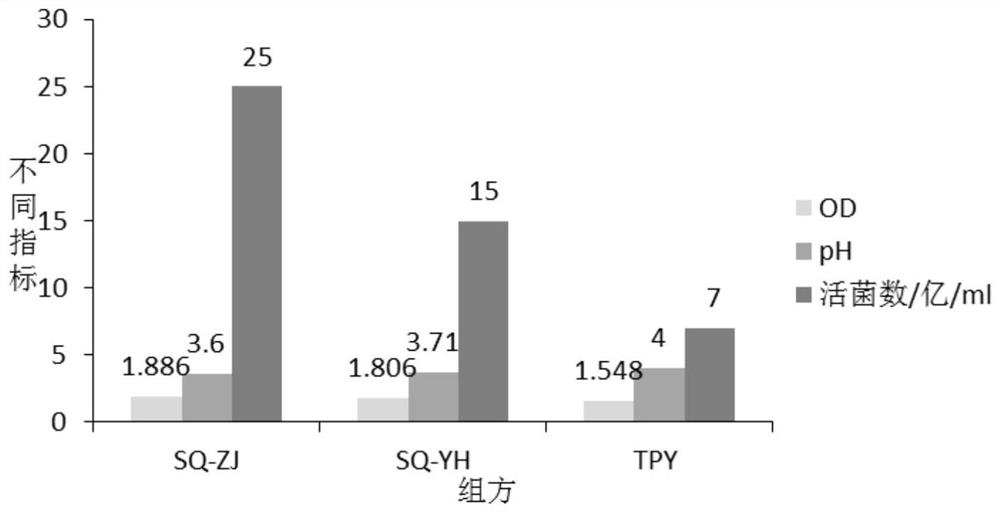
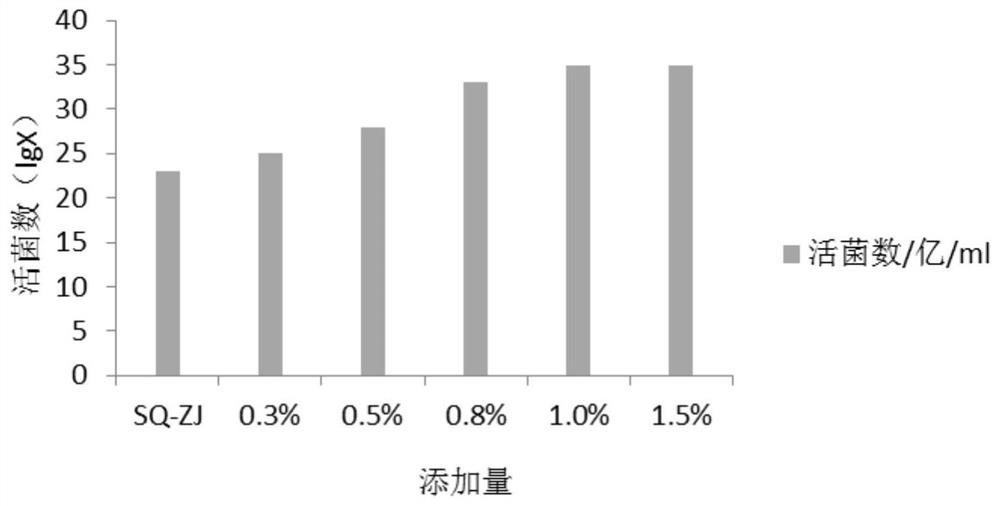
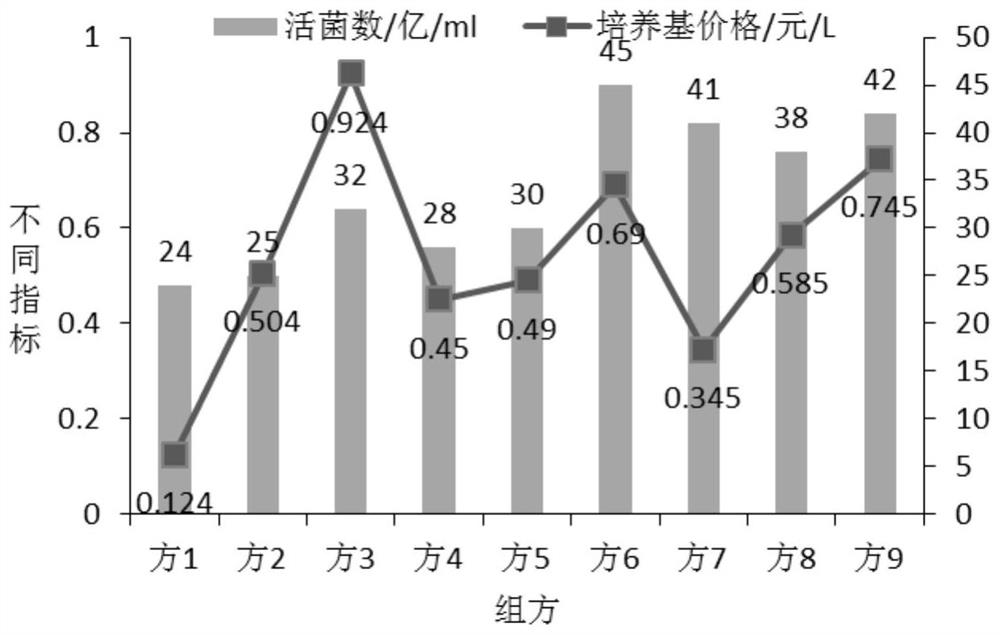
[0063] 1. The influence of different carbon and nitrogen sources on the number of live bacteria fermented by mixed strains:
[0064] According to the analysis of the lactobacillus and bifidobacterium formulations involved in the current workshop and literature, the main components of the medium are glucose, lactose, fructooligosaccharides, peptone, soybean peptone, yeast powder, and beef extract, and an orthogonal test with 7 factors and 2 levels is carried out , and the results of the percentage content of the 7 factors of serial numbers 1-8 are orthogonally obtained, and the serial numbers 1-8 are weighed according to the percentage content of the 7 factors in turn, and then the basic medium components are added. The basic medium components are: dipotassium hydrogen phosphate 1.35%, sulfuric acid Magnesium 0.02%, Tween 0.1%, L-cysteine hydrochloride 0.05%, adjust pH6.8-7.0, prepare 8 kinds of c...
Embodiment 2
[0081] Embodiment 2, a kind of high-density mixed culture and large-scale production fermentation method of direct injection type bifidobacterium and lactobacillus, described method comprises the following steps:
[0082] Step 1, strain activation culture:
[0083] The MRS and TPY slant medium were adjusted to pH 6.5 with 6mol / L sodium hydroxide, and sterilized at 121°C for 15 minutes by moist heat for use; The ampoules of bifidobacteria were placed at room temperature for 2 hours, and then the surface of the ampoule was wiped with 75% alcohol cotton balls in the aseptic operating table, and the ampoule was opened, and 2-3 rings were picked with sterilized inoculation loops, and evenly scratched. Line, Lactobacillus plantarum was inoculated on MRS slant medium, Bifidobacterium longum was inoculated on TPY slant medium, and cultured at 30°C under anaerobic conditions for 48 hours, the culture was terminated, and the morphology of bacteria was checked under the microscope. Acti...
Embodiment 3
[0102] Embodiment 3, a kind of high-density mixed culture and large-scale production fermentation method of direct injection type bifidobacterium and lactobacillus, described method comprises the following steps:
[0103] Step 1, strain activation culture:
[0104]Adjust the pH value of the MRS and TPY slant medium to 7.0, and sterilize at 121°C for 30 minutes before use; The ampoules were placed at room temperature for 3 hours, and then the surface of the ampoule was wiped with 75% alcohol cotton balls in the aseptic operation table, the ampoule was opened, and 2-3 loops were picked with a sterilized inoculation loop, and the lines were drawn evenly. Lactobacillus lysus was inoculated on the MRS slant medium, and Bifidobacterium lactis was inoculated on the TPY slant medium, cultured under anaerobic conditions at 37°C for 24 hours, the culture was terminated, the morphology of the bacteria was checked under the microscope, and continuous activation after no pollution 2 Secon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com