Transgenic zebrafish expressing gene Cas9 and construction method and application of transgenic zebrafish
A construction method and zebrafish technology, applied in the field of transgenic research, can solve the problems of difficult, time-consuming and labor-intensive knockout fish
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] (1) Preparation of sgRNA template
[0031] System: 5×Buffer5μL,
[0032] dNTP (10mM) 0.5 μL,
[0033] sgRNA-F (μM) 1 μL,
[0034] sgRNA-R (μM) 1 μL,
[0035] DNA Polymerase 0.5 μL,
[0036] RNase-free water 17μL,
[0037] Total25μL.
[0038] Conditions: 98°C for 2 minutes, 50°C for 10 minutes, and 72°C for 10 minutes.
[0039] (2) Product recovery
[0040] Take 2 μL of the product and use 2.5% agarose gel for electrophoresis detection.
[0041] (3) sgRNA in vitro transcription
[0042]
[0043] Conditions: overnight at 37°C.
[0044] (4) In vitro purification (removal of free bases, T7 enzyme, template DNA)
[0045] 1) Add RNase-free water to the sgRNA in vitro transcription product to make up to 100 μL. Add an equal volume (100 μL each) of Tris-saturated phenol-chloroform, centrifuge at 15,000 rpm at 4°C for 10 min, and slowly absorb the supernatant;
[0046] 2) Add 1 mL of 100% ethanol, shake for 10 seconds, centrifuge at 15,000 rpm at 4°C for 10 minutes,...
Embodiment 1
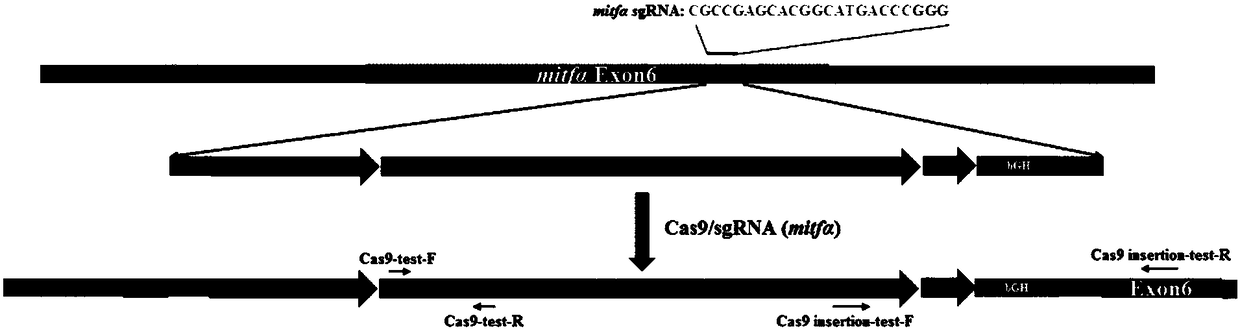
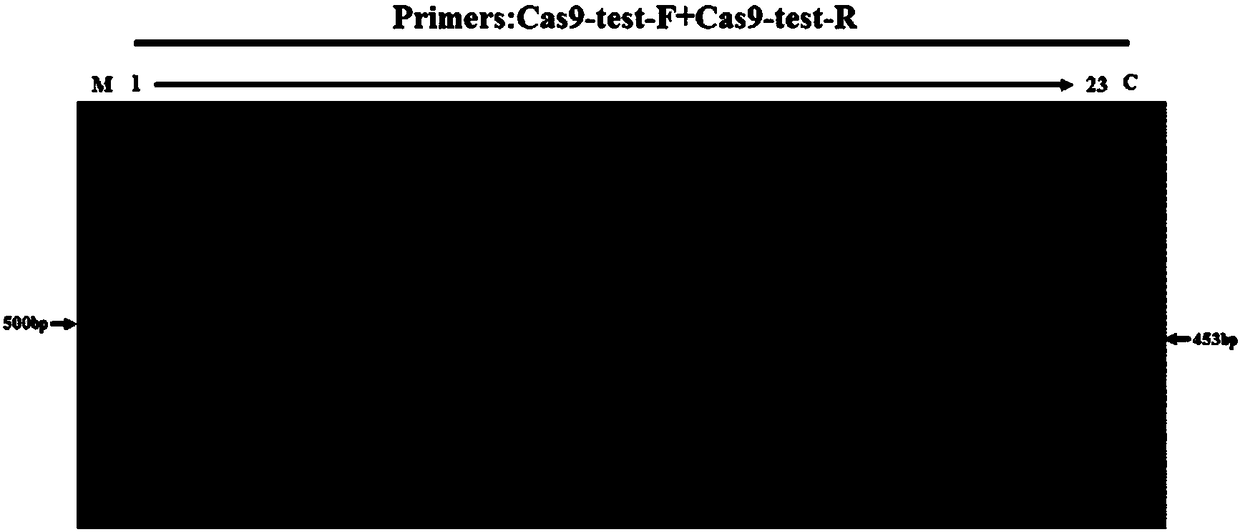
[0058] In Example 1, the zebrafish Mitfα gene was used as the Cas9 gene insertion site, and a mixture of Cas9 mRNA, MitfasgRNA, and Cas9 gene donor fragment was injected into fertilized eggs by microinjection. The integration of the Cas9 gene in the F0 generation of zebrafish was identified, and its expression in the offspring (F1) was detected, and finally the homozygous line was selected in the F1 generation by using pigment extinction.
[0059] Specifically include the following steps:
[0060] (1) Scientifically raise zebrafish, select healthy male fish and female fish, produce fertilized eggs through natural mating (light cycle: 14h light, 10h dark, male to female ratio 2:1), and collect fertilized eggs.
[0061] (2) The zebrafish Mitfα gene was used as the Cas9 gene integration site to synthesize Mitfα sgRNA and Cas9 mRNA, respectively. The Ef1α promoter of zebrafish itself was combined with the front end of the Cas9 gene in the MLM3613 vector, and the recombinant plasm...
Embodiment 2
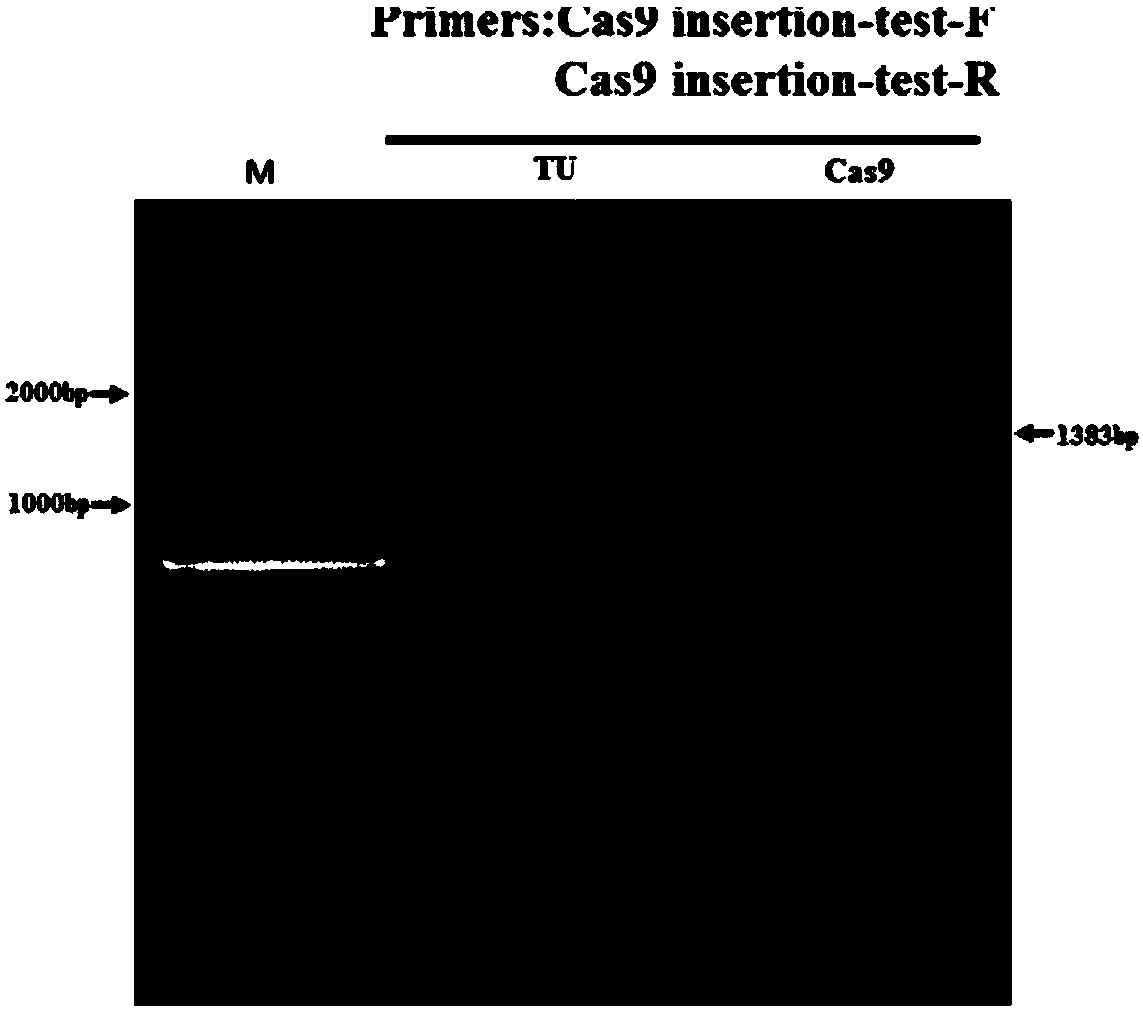
[0079] In Example 2, by editing the Tyr gene, it was proved that the constructed zebrafish expressing the Cas9 gene can be used as a gene editing tool. Embryos of transgenic Cas9 zebrafish and wild-type TU zebrafish were used for microinjection, and a control group was set up. The samples of injection group at 48hpf development were collected, and genomic DNA was extracted for identification.
[0080] Specifically include the following steps:
[0081] (1) Scientifically raise zebrafish, select healthy wild zebrafish and Cas9 transgenic zebrafish males and females, respectively, and naturally mate and reproduce to produce fertilized eggs (light cycle: 14h light, 10h dark, male to female ratio 2:1 ), and fertilized eggs were collected separately. A control group (no injection), a traditional method embryo injection group, and a Cas9 transgenic zebrafish injection group were set up. In addition, some transgenic zebrafish embryos were left without any treatment.
[0082] (2) F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com