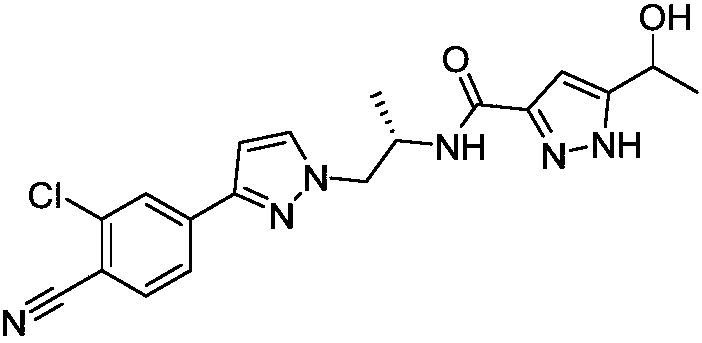
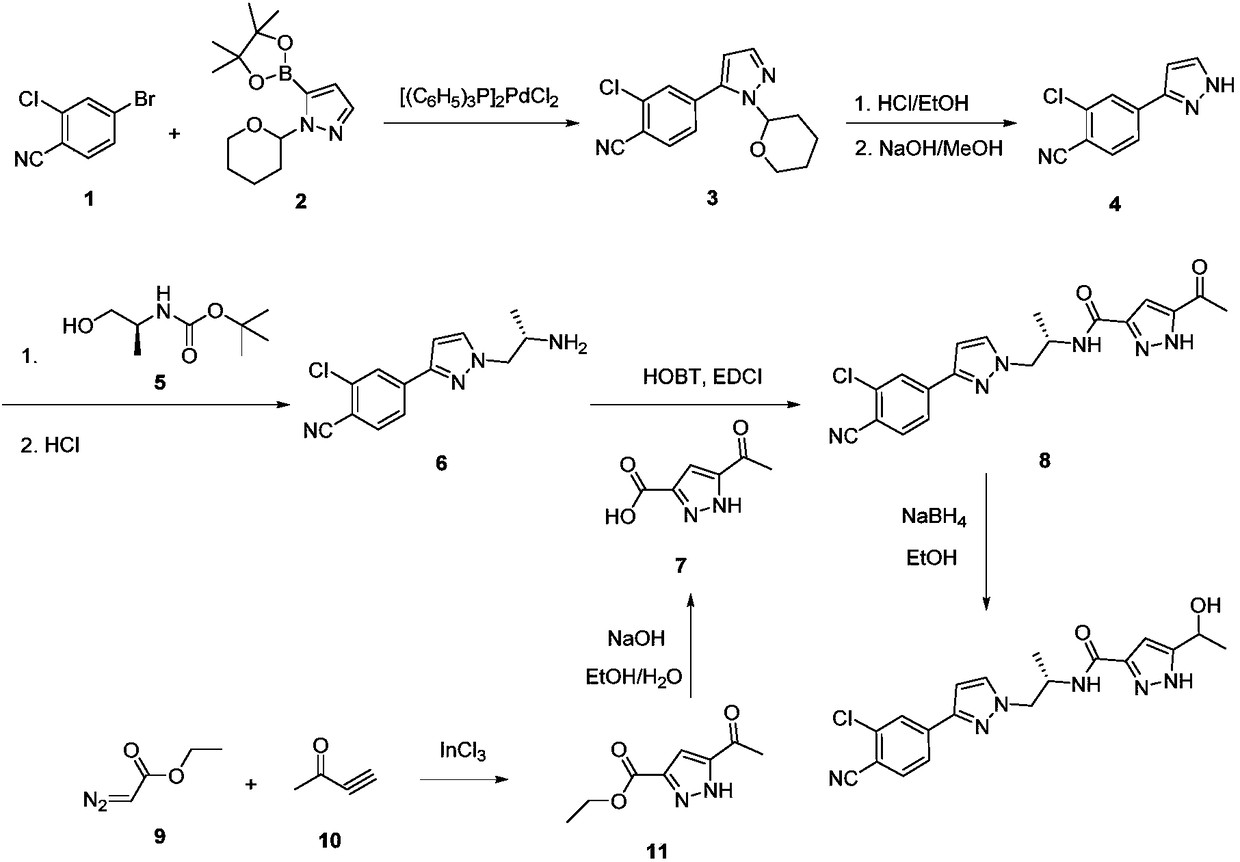
Method for preparing novel androgen receptor antagonist
A technology of solvents and compounds, applied in the field of drug synthesis, can solve problems such as unsuitable for industrial production, unsuitable for industrial production, and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: (R)-3-((tert-butyldimethylsilyl)oxy)butanoic acid methyl ester
[0135]
[0136] Add (R)-3-hydroxybutyric acid methyl ester (compound of formula Xa) (20g, 0.17mol), imidazole (23g, 0.34mol), anhydrous dichloromethane 350mL into a 500mL three-necked flask in sequence, replace with nitrogen three times, and cool down the system To 0°C, TBSCl (30.5 g, 0.2 mol) was slowly added dropwise, and the mixture was stirred at room temperature for 3 h after the drop was completed. After the reaction was completed, the reaction solution was poured into 300 mL of ice water to quench, the organic layer was washed three times with water and saturated brine three times, dried over anhydrous sodium sulfate, filtered, and the organic layer was concentrated under reduced pressure and evaporated to dryness to obtain 38 g of the title compound with a yield of 96 %.
Embodiment 2
[0137] Example 2: (R)-3-((tert-butyldimethylsilyl)oxy)butyraldehyde
[0138]
[0139] Add (R)-3-((tert-butyldimethylsilyl)oxy)methyl butyrate (15g, 64mmol) and 375mL of anhydrous dichloromethane successively into a 500mL three-necked flask, replace with nitrogen three times, and cool the system to -78°C, slowly drop into DIBAL-H (67.7mL, 1.0mol / L dissolved in n-hexane, 67mmol), and stir for 1h after dropping. After the reaction, add 15mL methanol to quench the reaction, then pour the reaction solution into 200mL saturated sodium potassium tartrate aqueous solution, stir, add 300mL diethyl ether to extract three times, wash the organic layer three times with water, wash three times with saturated saline, anhydrous sodium sulfate Dry, filter, and concentrate the organic phase under reduced pressure to obtain 12 g of the title compound, with a yield of 92%.
Embodiment 3
[0140] Example 3: (5R)-5-((tert-butyldimethylsilyl)oxy)-2-diazo-3-hydroxy-hexanoic acid ethyl ester
[0141]
[0142] Add (R)-3-((tert-butyldimethylsilyl)oxy)butyraldehyde (10g, 49mmol), ethyl diazoacetate (8.9g, 78mmol) and 300mL of anhydrous THF to a 500mL three-necked flask in sequence , replaced with nitrogen three times, the system was cooled to -78°C, and LIHMDS (74mL, 1.0mol / L dissolved in THF, 74mmol) was slowly added dropwise, and stirred for 1h after the drop was completed. After the reaction was completed, add 100 mL saturated ammonium chloride aqueous solution to quench the reaction, stir to room temperature, extract the solution three times with 300 mL ethyl acetate, combine the organic layers, wash three times with saturated saline, dry over anhydrous sodium sulfate, filter on silica gel, and concentrate under reduced pressure 13 g of the title compound were obtained with a yield of 83.3%.
[0143] ESI-MS(m / z):317[M+H] + ;
[0144] 1 H-NMR (400MHz, CDCl 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com