Continuous preparation method of DL-muscone
A technology of muskone and continuous chromatography, which is applied in the field of continuous preparation of DL-musketone, can solve the problems of low reactor capacity, difficulty in low-cost production, and large amount of solvent usage, and achieve high process stability, continuous production, and high-quality products. The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A continuous preparation method of DL-Muscone, comprising the following steps:
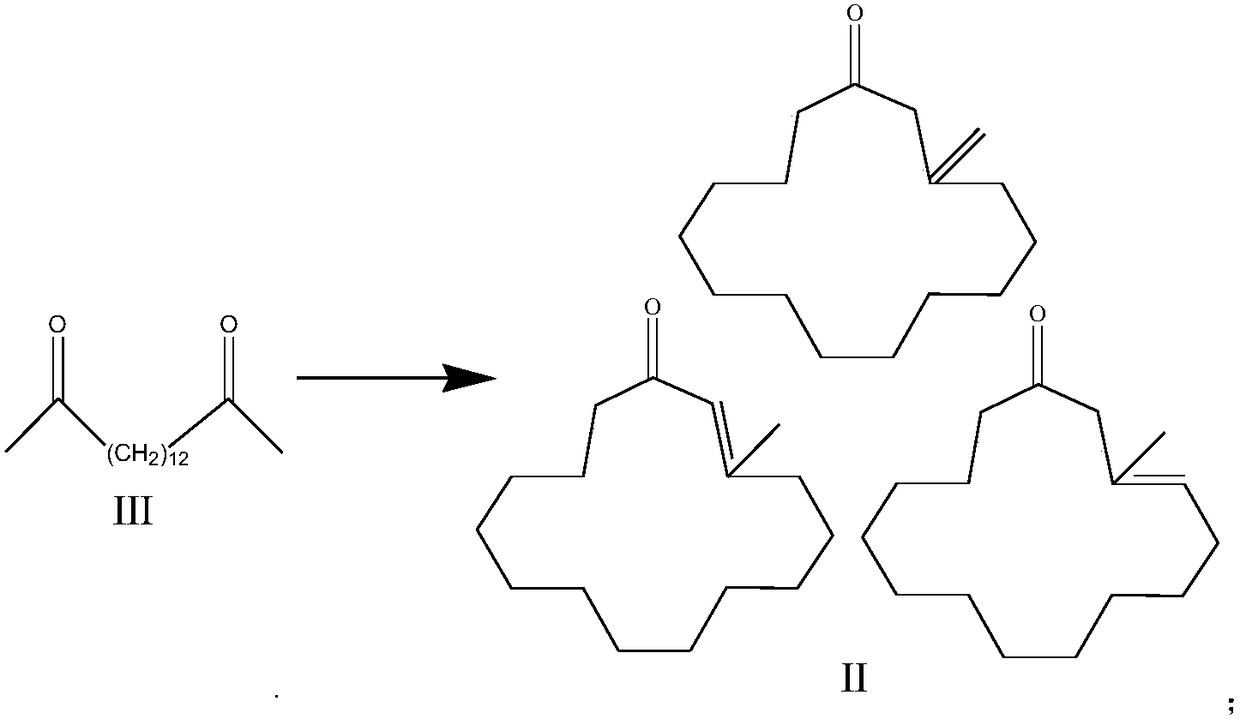
[0027] 1) Dissolve 2,15-hexadecanedione III in an aprotic solvent, then react continuously in a fixed-bed cyclization reactor, and cyclize under the action of a cyclization catalyst to generate 3-methylcyclopentacene Ketone analog II;
[0028]
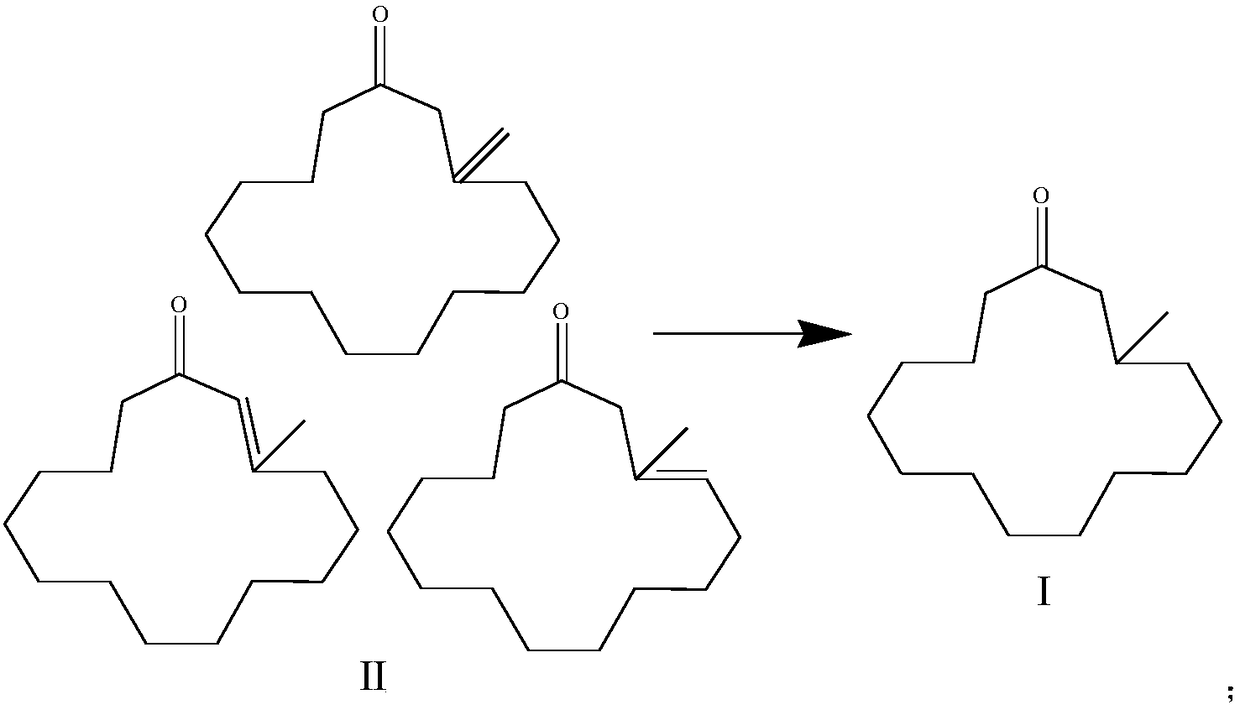
[0029] 2) After the 3-methylcyclopentacenone analog II is desolvated, it is dissolved in a protic solvent, and then reacted continuously with hydrogen in a fixed-bed hydrogenation reactor under the action of a hydrogenation catalyst to obtain the formula Muscone shown in Ι;
[0030]
[0031] 3) Precipitate the product obtained in step 2) and carry out continuous chromatographic separation, the product solution containing DL-musketone undergoes desolventization to obtain the DL-muscarone product, and the component containing 2,15-hexadecanedione is decolorized Return to step 1) for use after purification.
[0032] In the present invention, 2...
Embodiment l
[0046]2,15-hexadecanedione III is dissolved in dichloroethane (the weight ratio of dichloroethane to 2,15-hexadecanedione is 10:1), and then passed through a fixed-bed cyclization reactor continuous response. The ring closure catalyst is SiO 2 supported nano ZrO 2 , the weight hourly space velocity of 2,15-hexadecanedione on the catalyst is 0.01h -1 . The catalyst bed temperature is 300°C, and the vacuum degree is 0.06MPa.
[0047] Precipitate the 3-methylcyclopentacenone analog II obtained after cyclization, then dissolve it in absolute ethanol and react continuously with hydrogen in a fixed-bed hydrogenation reactor, ethanol and 3-methylcyclopentacenone The weight ratio of pentacenone is 10:1. The hydrogenation catalyst is palladium supported on activated carbon, the palladium load is 1%, and the weight hourly space velocity (calculated as 3-methylcyclopentacenone analogue) is 0.01h -1 . The reaction temperature is 90° C., and the hydrogen pressure is 3 MPa.
[0048]...
Embodiment 2
[0050] 2,15-hexadecanedione III was dissolved in chloroform (the weight ratio of chloroform to 2,15-hexadecanedione was 4:1), and then reacted continuously in a fixed-bed cyclization reactor. The ring closure catalyst is Al 2 o 3 supported nano ZrO 2 , the weight hourly space velocity of 2,15-hexadecanedione on the catalyst is 0.1h -1 . The catalyst bed temperature is 280°C, and the vacuum degree is 0.07MPa.
[0051] The 3-methylcyclopentacenone analog II obtained after cyclization is desolvated, then dissolved in anhydrous methanol and continuously reacted with hydrogen in a fixed-bed hydrogenation reactor, methanol and 3-methylcyclopentacenone The weight ratio of pentacenone analogs is 5:1. The hydrogenation catalyst is palladium supported on activated carbon, the palladium load is 2%, and the weight hourly space velocity (calculated as 3-methylcyclopentacenone analogue) is 0.2h -1 . The reaction temperature is 60° C., and the hydrogen pressure is 2.5 MPa.
[0052] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com