Metal organic gold (iii) complexes and their synthesis methods and applications
A synthesis method and compound technology, applied in the field of medicine, can solve the problems that have not been found in activity research, and achieve the effects of cheap and easy-to-obtain reaction raw materials, good proliferation inhibitory activity, resistance to drug resistance, and high physiological stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
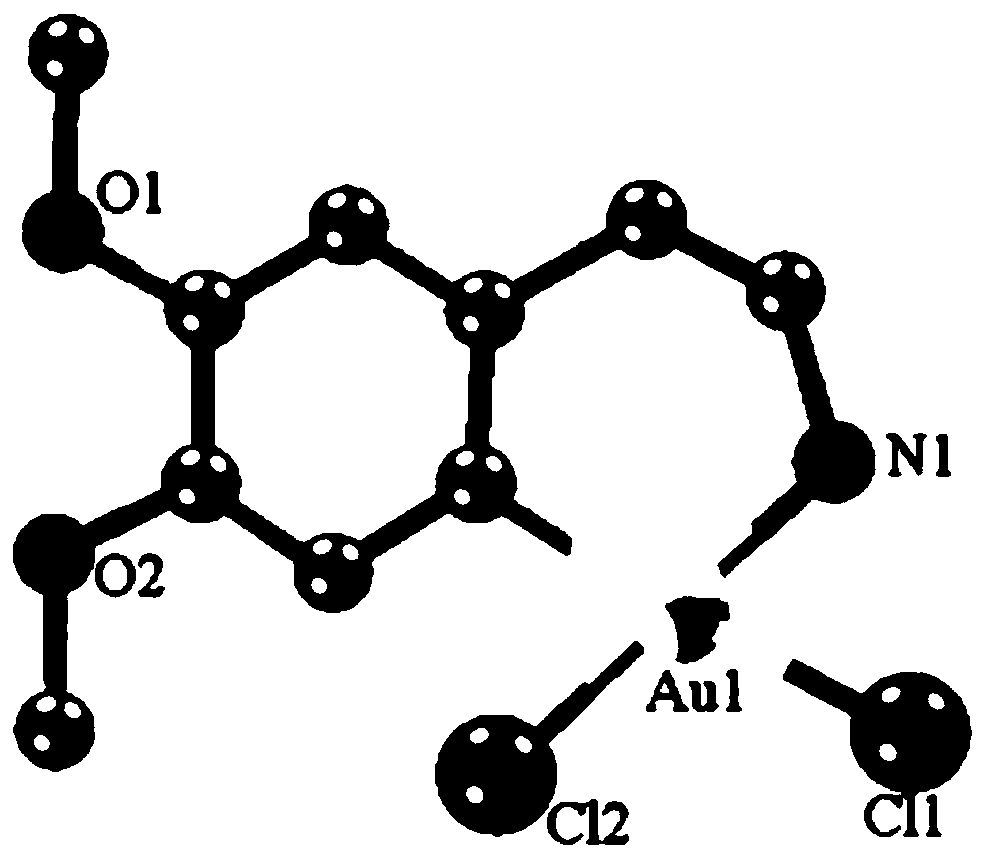
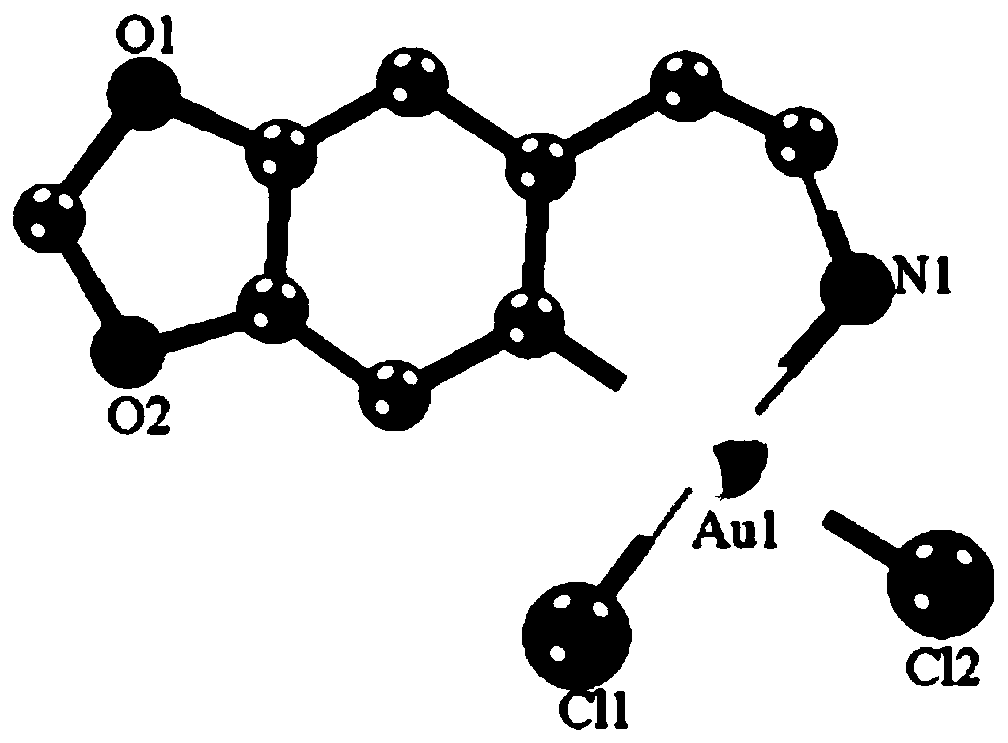
[0021] Embodiment 1: the synthesis of target product Cyc-Au-1
[0022] Take KAuCl 4 (0.2mmol) and 3,4-dimethoxyphenethylamine (0.2mmol) are placed in the container, then add 30mL in the mixed solvent that is made up of dichloromethane and methanol (the volume ratio of dichloromethane and methanol is 1 : 1), the resulting mixture was refluxed and stirred for 24 hours under dark conditions, the resulting reactant was filtered, the filtrate was collected, and left to stand, yellow crystals were precipitated, the crystals were collected, and vacuum-dried to obtain a yellow solid product with a yield of 55%.
[0023] The product obtained in this embodiment is characterized:
[0024] 1) proton nuclear magnetic spectrum analysis, the obtained spectral data are as follows:
[0025] H NMR spectrum: 1 H NMR (400MHz, DMSO-d 6 )δ6.84(d,J=8.1Hz,1H),6.70(dd,J=8.1,1.6Hz,1H),3.74(s,3H),3.72(s,3H),2.81–2.67(t,J =5.4Hz 2H), 2.57(t, J=7.2Hz, 2H).
[0026] 2) Electrospray mass spectrometry ...
Embodiment 2
[0034] Embodiment 2: the synthesis of target product Cyc-Au-1
[0035] Example 1 was repeated except that the mixed solvent consisting of dichloromethane and methanol was replaced with chloroform. Yield 58%.
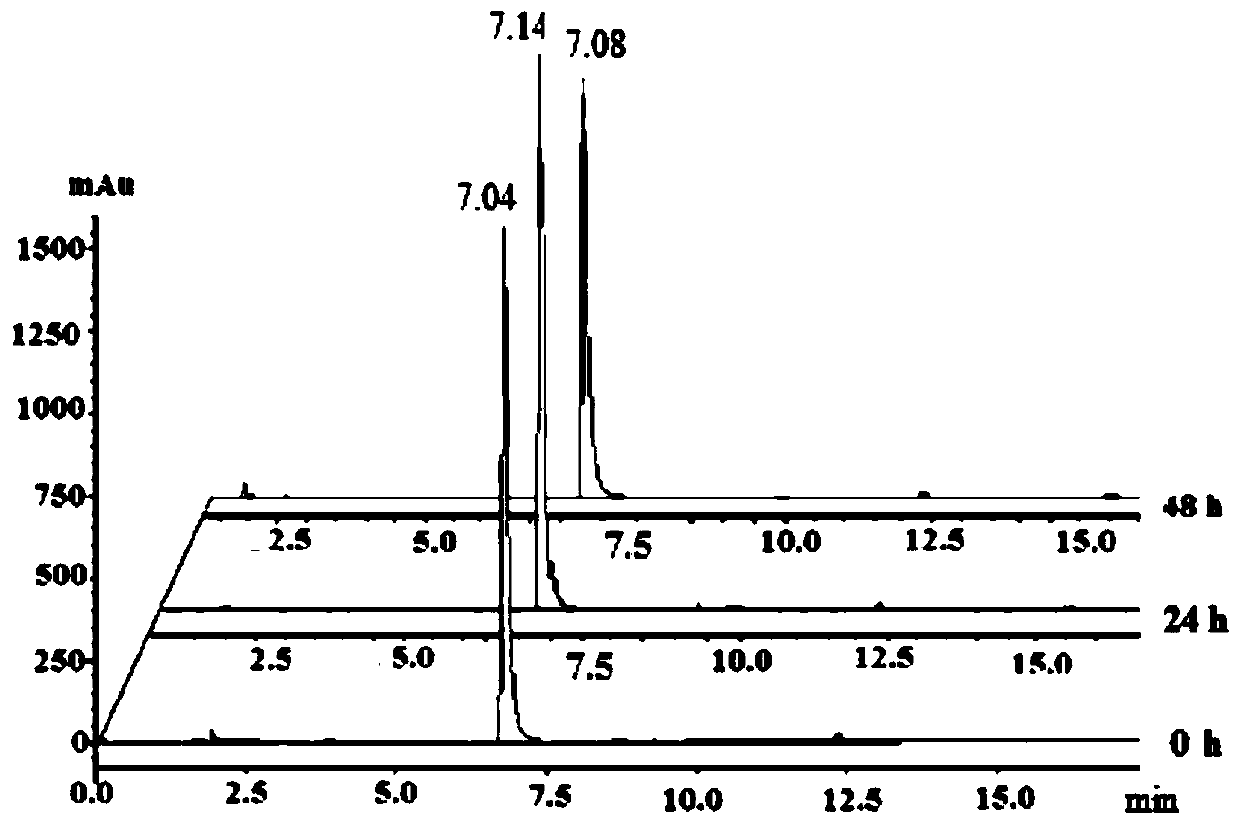
[0036] The product obtained in this example was analyzed by proton nuclear magnetic spectrum, electrospray mass spectrometry and X-ray single crystal diffraction, and it was determined that the product obtained in this example was the target product Cyc-Au-1.
Embodiment 3
[0037] Embodiment 3: the synthesis of target product Cyc-Au-1
[0038] Example 1 was repeated, except that the reaction was carried out at 25° C., and the reaction time was 48 hours. Yield 46%.
[0039] The product obtained in this example was analyzed by proton nuclear magnetic spectrum, electrospray mass spectrometry and X-ray single crystal diffraction, and it was determined that the product obtained in this example was the target product Cyc-Au-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com