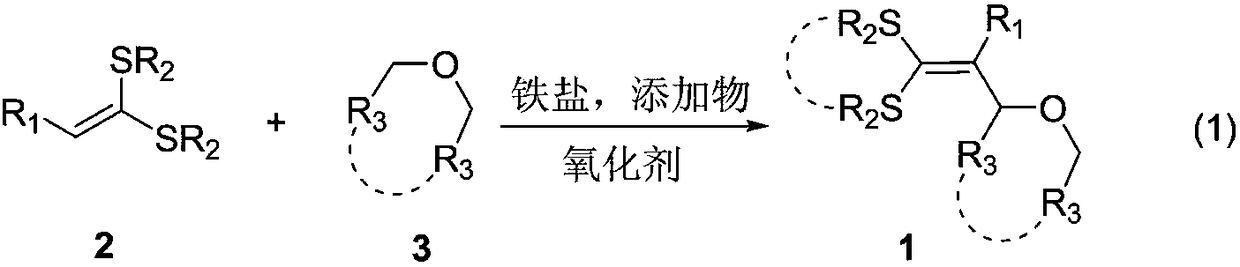
3-alkoxy-1,1-dialkylthiol propylene derivative and synthetic method
A technology of dialkylthiopropene and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low atom utilization rate, few alkenes, and narrow application range of substrates, so as to avoid pre-functionalization, simplify synthesis steps, and achieve application scope. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
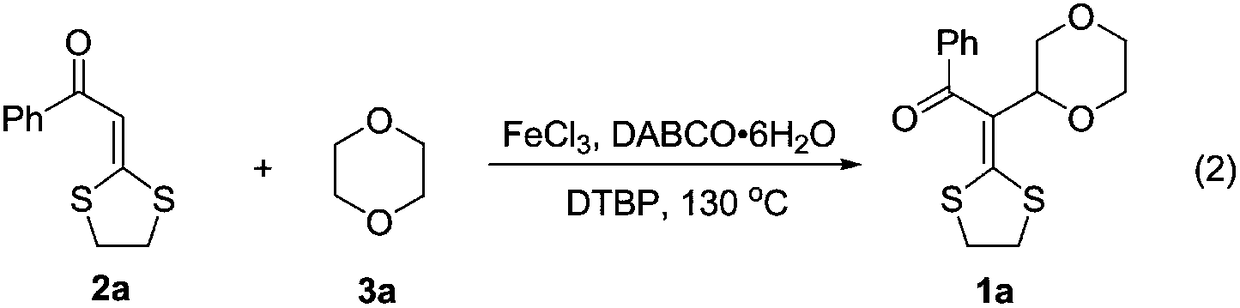
Embodiment 1
[0028]
[0029] The specific process is: weigh dithioketene 2a (111.2 mg, 0.5 mmol), FeCl 3 (4.1mg, 0.025mmol), DABCO·6H 2 O (11.0mg, 0.05mmol) was added to a 25mL sealed tube with a branch, 1,4-Dioxane (3mL) and DTBP (219.2mg, 1.5mmol) were added under a nitrogen atmosphere, and placed in an oil bath at 130°C React in 24h. After the reaction is complete, cool to room temperature, filter with diatomaceous earth, and rotary evaporate under reduced pressure to remove the solvent, then column chromatography (petroleum ether (60-90°C) / ethyl acetate: 8:1, v / v) to obtain a light yellow Solid product 1a (122.5 mg, yield 80%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0031]
[0032] The reaction steps and operations are the same as in Example 1, except that the dithioketene added to the reaction system is 2b (150.0 mg, 0.5 mmol). The reaction was stopped, and the target product 1b (146.0 mg, yield 76%) was obtained as a light yellow solid after post-processing. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0034]
[0035] The reaction steps and operations are the same as those in Example 1, except that the dithioketene added to the reaction system is 2c (118.0 mg, 0.5 mmol). The reaction was stopped, and the target product 1c (128.0 mg, yield 79%) was obtained as a light yellow solid after post-processing. The target product was confirmed by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com