Synthesis method of 4-alkoxyphenol compound
A technology of alkoxyphenol and synthesis method, which is applied in the field of organic chemical synthesis, can solve problems such as limited application and narrow substrate range, and achieve the effects of easy separation and purification, high practicability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
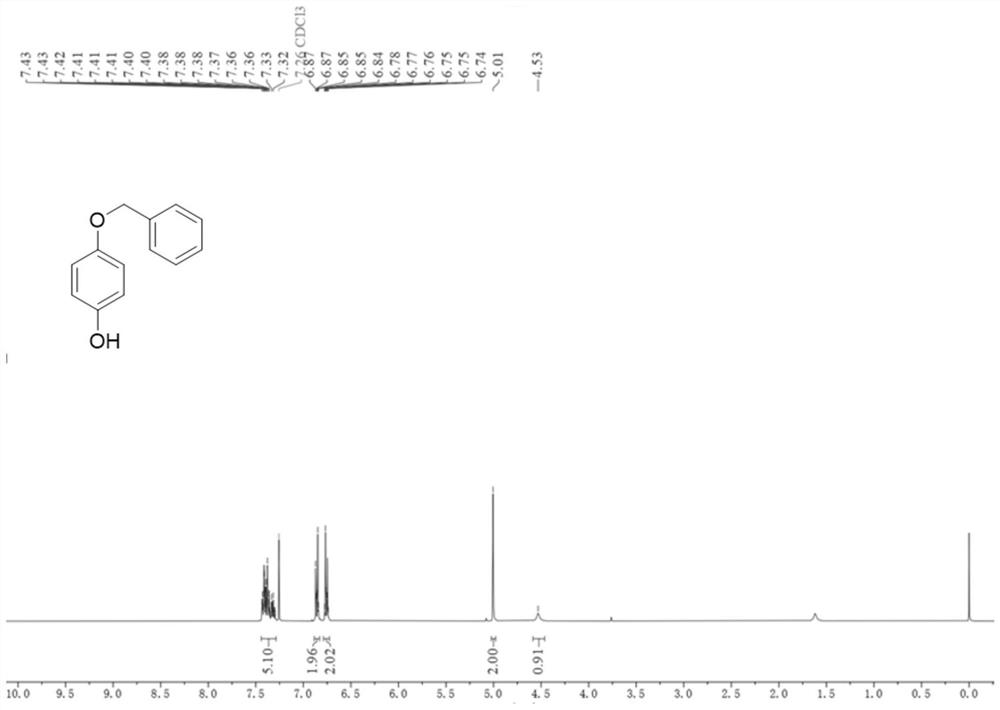
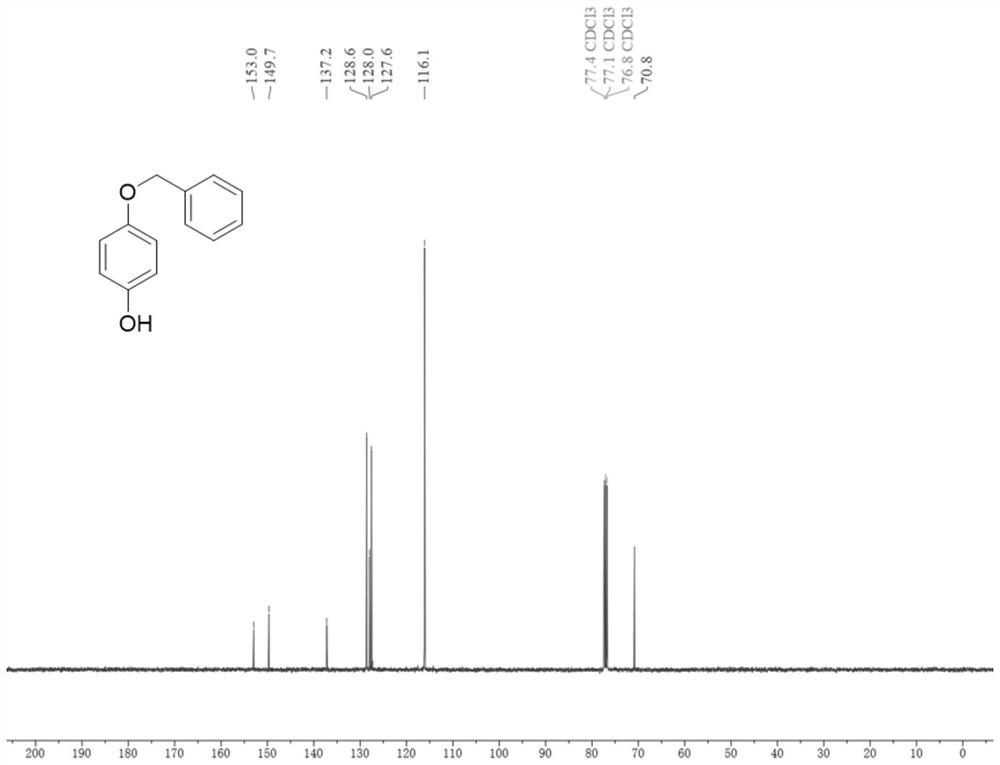
[0040] Add compound 1a (9mmol), rhodium dipolyacetate (0.135mmol) and iodophendiethyl (9mmol) sequentially into a 75mL pressure tube, then add trifluoroacetic anhydride (30mL), and finally place in an oil bath at 80°C The reaction was carried out under medium magnetic stirring for 1.5 hours. After the reaction was completed, the reaction solution was transferred to a round-bottomed flask, and trifluoroacetic anhydride was distilled off at 70°C, then extracted with ethyl acetate (60mL×3), the organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate , the solvent was distilled off under reduced pressure and separated by column chromatography (petroleum ether: ethyl acetate = 8:1, v / v) to obtain the white solid product 2a with a yield of 80%. The compound characterization data are as follows: 1 HNMR (400MHz, Chloroform-d) δ7.44–7.29(m,5H),6.88–6.83(m,2H),6.79–6.73(m,2H),5.01(s,2H),4.53(s,1H) ; 13 C NMR (101MHz, Chloroform-d) δ153.0, 14...
Embodiment 2
[0042]
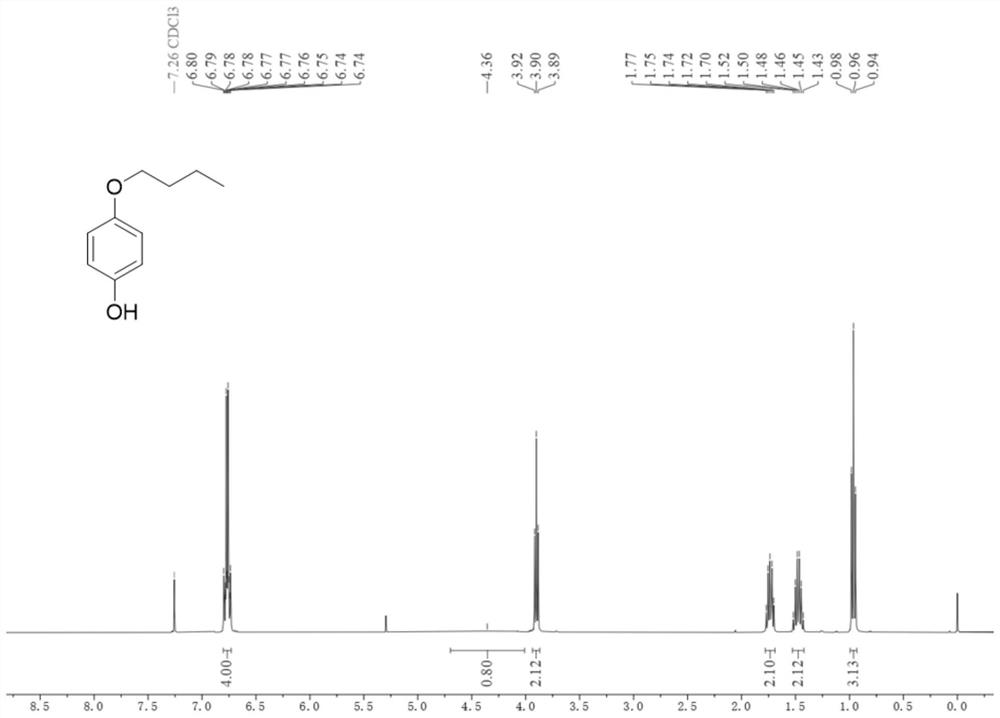
[0043] Add compound 1b (10mmol), rhodium dipolyacetate (0.15mmol) and iodophendiethyl (10mmol) sequentially into a 75mL pressure tube, then add trifluoroacetic anhydride (30mL), and finally place in an oil bath at 80°C The reaction was carried out under medium magnetic stirring for 1 hour. After the reaction was completed, the reaction solution was transferred to a round-bottomed flask, and trifluoroacetic anhydride was distilled off at 70°C, then extracted with ethyl acetate (60mL×3), the organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate , the solvent was distilled off under reduced pressure, and separated by column chromatography (petroleum ether: ethyl acetate = 10:1, v / v) to obtain 2b as a light brown solid with a yield of 95%. The compound characterization data are as follows: 1 HNMR (400MHz, Chloroform-d) δ6.80–6.73(m,4H),4.36(s,1H),3.90(t,J=6.5Hz,2H),1.78–
[0044] 1.69(m, 2H), 1.47(h, J=7.4Hz, 2H), 0.96(t, J=7.4Hz, 3H)...
Embodiment 3
[0047]
[0048] Add compound 1c (0.3mmol), dipolyrhodium acetate (0.45%mmol) and iodobenzenediethyl ester (0.3mmol) successively into a 10mL pressure tube, then add trifluoroacetic anhydride (1.5mL), and finally place Magnetic stirring was carried out in an oil bath at 80°C for 1 hour. After the reaction was completed, 15ml of the reaction was quenched with saturated sodium bicarbonate, then extracted with ethyl acetate (20mL×3), the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and column chromatography separated (Petroleum ether: ethyl acetate = 10:1, v / v) the beige solid product 2c was obtained with a yield of 85%. The compound characterization data are as follows: 1 H NMR (400MHz, Chloroform-d) δ6.81–6.74(m, 4H), 4.71(s, 1H), 3.86(t, J=6.6Hz, 2H), 1.78(h, J=7.1Hz, 2H) ,1.02(t,J=7.4Hz,3H); 13 C NMR (101MHz, Chloroform-d) δ153.2, 149.4, 116.1, 115.7, 70.4, 22.7, 10.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com