Asymmetric N-pyridyl-2,2-diindyl compound and synthetic method thereof
A technology of pyridyl indole compound and indole compound, applied in the direction of organic chemistry, can solve the problems of harsh multi-step operation, difficult synthesis, difficult pre-functionalization, etc., and achieve high purity, high yield and narrow melting point range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
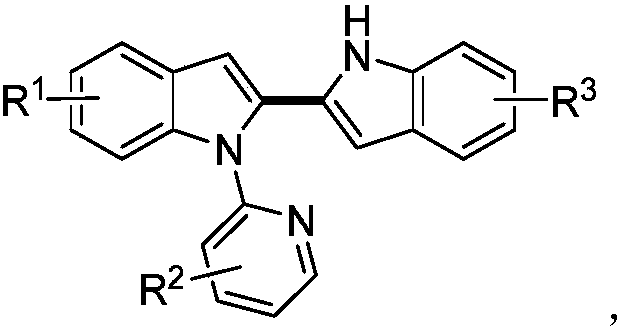
[0035] A kind of unsymmetrical N-pyridyl-2,2-indole compound, its general chemical structure formula is:
[0036]
[0037] The synthetic method of above-mentioned unsymmetrical N-pyridyl-2,2-indole compound, comprises the following steps:
[0038] (1) Under an air atmosphere, mix 0.2 mmol of N-pyridyl indole compound, 0.4 mmol of indole, [Cp*Co(CO)I 2 ]0.005mmol, AgSbF 6 0.02mmol, HOPiv 0.04mmol, Ag 2 O 0.4mmol, NaOAc 0.2mmol and 1,2-dichloroethane 2ml were mixed in a reaction tube, and heated in an oil bath at 100°C for 15h;
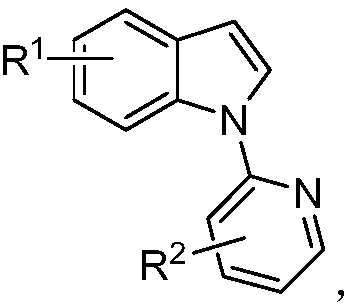
[0039] Described N-pyridyl indole compound structural formula is:
[0040] (2) After the reaction is completed, the reaction tube is cooled to room temperature, the reaction mixture is desalted, washed, concentrated, and purified to obtain the target product N-pyridyl-2,2-indole compound with a yield of 86% and a melting point of 190 -192°C. 1 H NMR (400MHz, CDCl 3 )δ8.95(br,1H),8.73(d,J=3.6Hz,1H),7.78(td,J=7.8,1.7Hz,1H),7.71–7.64(m,1H),7.53(...
Embodiment 2
[0045] A kind of unsymmetrical N-pyridyl-2,2-indole compound, its general chemical structure formula is:
[0046]
[0047] The synthetic method of above-mentioned unsymmetrical N-pyridyl-2,2-indole compound, comprises the following steps:
[0048](1) Under an air atmosphere, mix 0.2 mmol of N-pyridyl indole compound, 0.45 mmol of 6-methyl indole, [Cp*Co(CO)I 2 ]0.006mol, AgSbF 6 0.02mmol, HOPiv 0.05mmol, Ag 2 O 0.4mmol, NaOAc 0.22mmol and toluene 3ml were mixed in a reaction tube, and heated in an oil bath at 110°C for 11h;
[0049] Described N-pyridyl indole compound structural formula is:
[0050] (2) After the reaction was completed, the reaction tube was cooled to room temperature, and the reaction mixture was desalted, washed, concentrated, and purified to obtain the target product with a yield of 72% and a melting point of 194-196°C. 1 H NMR (400MHz, CDCl 3 ) δ8.93(br,1H),8.68–8.58(m,1H),7.66(td,J=7.8,1.9Hz,1H),7.59(dd,J=6.3, 2.6Hz,1H),7.45–7.37 (m,1H),7.25(dd...
Embodiment 3
[0052] A kind of unsymmetrical N-pyridyl-2,2-indole compound, its general chemical structure formula is:
[0053]
[0054] The synthetic method of above-mentioned unsymmetrical N-pyridyl-2,2-indole compound, comprises the following steps:
[0055] (1) Under an air atmosphere, mix N-pyridyl indole compound 0.2mmol, 5-chloroindole 0.5mmol, [Cp*Co(CO)I 2 ]0.0056mmol, AgSbF 6 0.022mol, HOPiv 0.044mol, Ag 2 O 0.42mmol, NaOAc 0.2mmol and toluene 2.2ml were mixed in a reaction tube, heated in an oil bath at 110°C for 13h;
[0056] Described N-pyridyl indole compound structural formula is:
[0057] (2) After the reaction was completed, the reaction tube was cooled to room temperature, and the reaction mixture was desalted, washed, concentrated, and purified to obtain the target product with a yield of 82% and a melting point of 180-182°C. 1 H NMR (400MHz, DMSO) δ11.76 (br, 1H), 8.71 (d, J = 3.6Hz, 1H), 8.02 (td, J = 7.7, 1.8Hz, 1H), 7.72 (dd, J = 5.4, 3.2Hz, 1H), 7.58(dd, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com