Styrene-based compound and preparation method and application thereof
A technology for compounds and bases, applied in the field of styrenyl compounds and their preparation, can solve problems such as drug resistance caused by treatment plans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
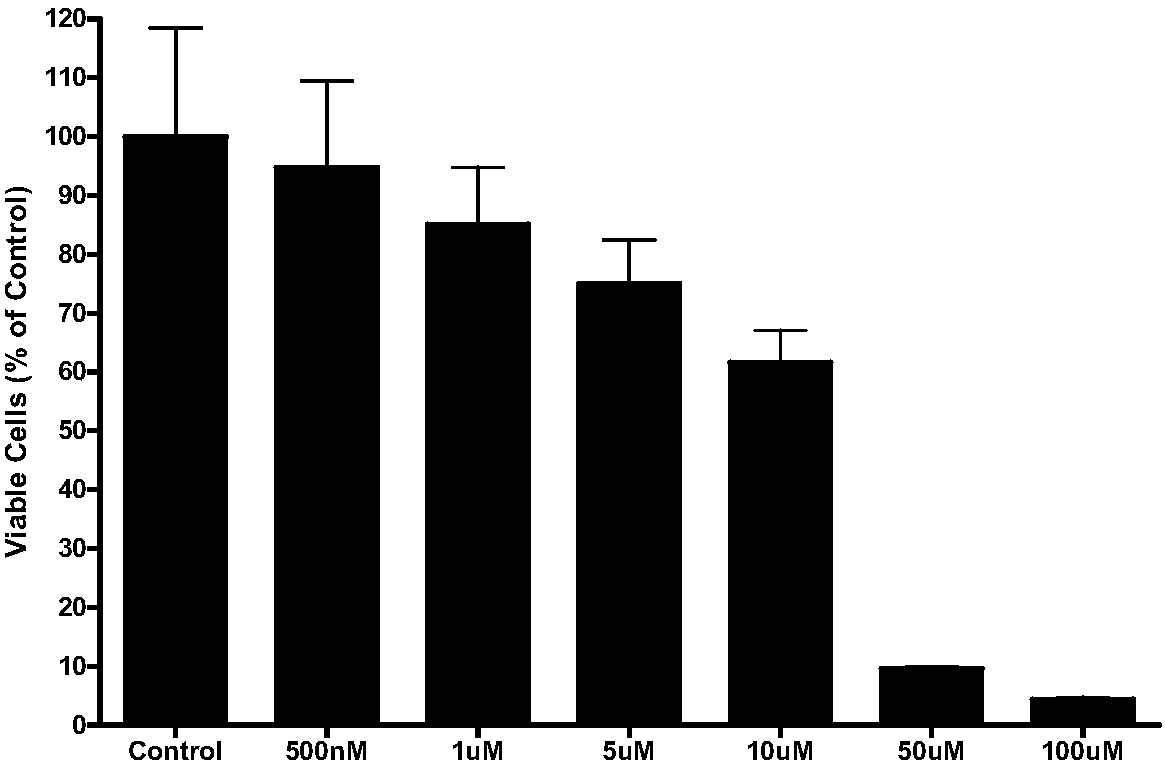
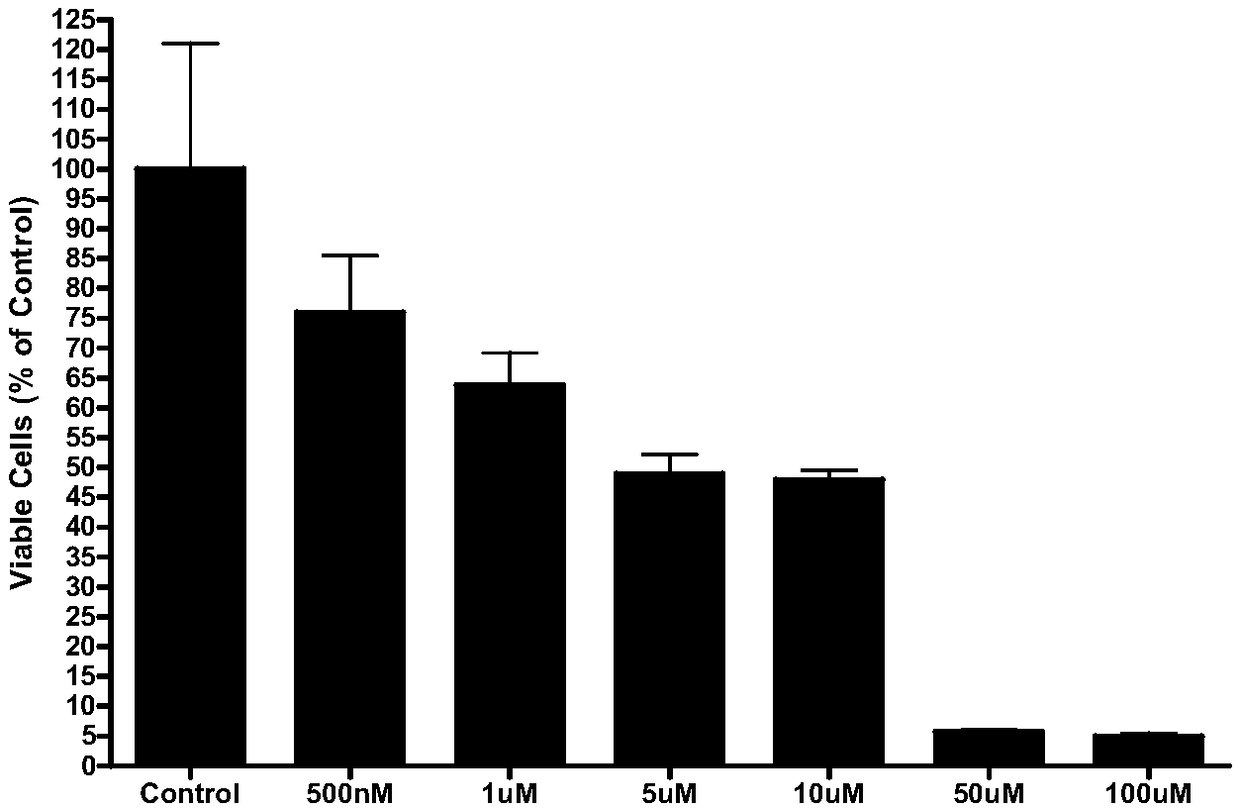
Image
Examples
Embodiment Construction
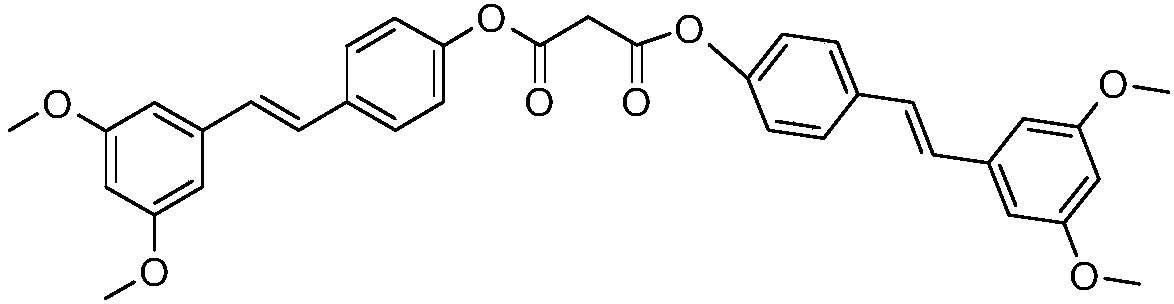
[0037] A styryl compound of the present invention, which at least contains 5-(4-(4-(3,5-dihydroxystyryl)phenoxy)styryl)-1,3-benzene of the following structural formula Diphenol compounds:
[0038]
[0039] The preparation reaction formula of the 5-(4-(4-(3,5-dihydroxystyryl) phenoxy) styryl)-1,3-benzenediol compound of the above structural formula is as follows:
[0040]
[0041] The 5-(4-(4-(3,5-dihydroxystyryl)phenoxy)styryl)-1,3-benzenediol compound of the above structural formula is prepared by the following method:
[0042] 1. Preparation of C9-1
[0043] The reaction formula is as follows:
[0044]
[0045] ①Feeding:
[0046]
[0047]
[0048] ②Operation and phenomenon:
[0049] Put 25.0g of SM1 into a 500ml single-necked bottle, add 250ml of DCM and stir to disperse evenly, then add 46.0g of thionyl chloride dropwise at room temperature, then add 5 drops of catalytic amount of DMF, and heat up to reflux after adding;
[0050] After stirring and react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com