Method for preparing methyl R-(+)-2-(4-hydroxyphenoxy)propionate
A technology of hydroxyphenoxy and methyl propionate, which is applied in the field of compound preparation, can solve the problems of reducing reaction efficiency, prolonging reaction time, and low reaction yield, achieving high reaction rate, reducing the amount of waste acid, and increasing yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
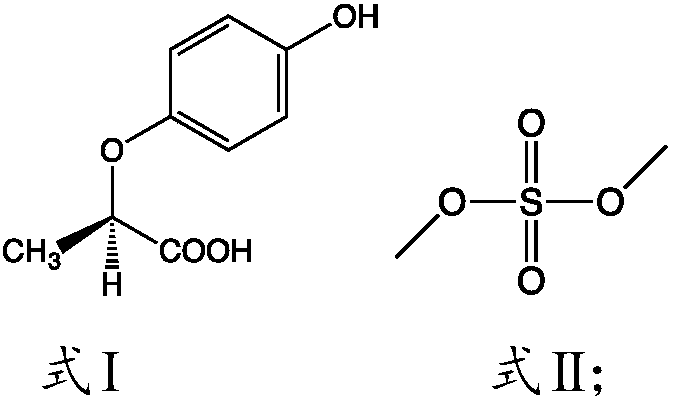
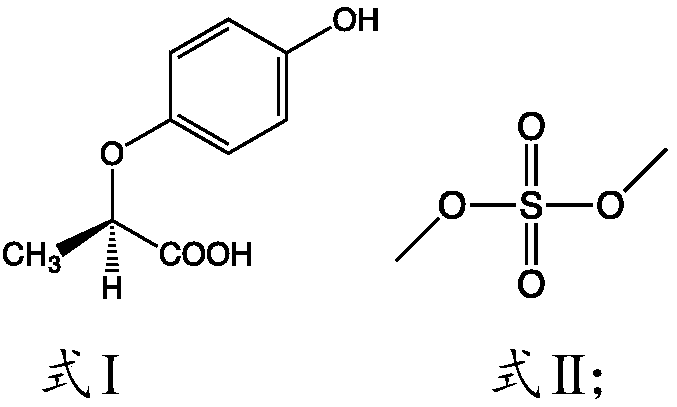
[0034] The present invention relates to the technical scheme of the present invention is to adopt a kind of preparation method of R-(+)-2-(4-hydroxyphenoxy) methyl propionate, comprising:
[0035] 1) Mix the compounds shown in formula I, methanol and formula II in a closed environment, and heat to reflux for esterification;
[0036]
[0037] According to the present invention, in order not to add catalyst in reaction process, and make reaction form catalyst, the present invention has preferably used the organic matter that contains sulfur, preferably uses dimethyl sulfate, in reaction process, a methyl alcohol and the compound shown in formula I carry out Esterification reaction, and the generated water can impel the hydrolysis of dimethyl sulfate to form sulfuric acid and methanol, and sulfuric acid and methanol can promote the esterification reaction of the compound shown in formula I and methanol to proceed forward, improving the conversion of the reaction Rate. Accordi...
Embodiment 1
[0059]Dimethyl sulfate (32g; 0.5eq), methanol (105.6g; 6eq) and R-(+)-2-(4-hydroxyphenoxy)propionic acid (91g; 1eq) were added in a 500mL reaction flask, and the The reaction was carried out under reflux for 4-6 hours, and the esterification conversion rate monitored by HPLC was greater than 99.5%, and the reaction was completed. Lower the temperature, start to concentrate methanol under reduced pressure at 30°C, control the vacuum degree <-0.095MPa, concentrate at below 55°C, recover the methanol for use, add toluene (182g, twice the raw material) until it cannot evaporate, and cool down to 30-40°C , continue to distill methanol under reduced pressure, detect methanol<1% (GC: area normalization method), wash the sulfuric acid produced by the reaction in the toluene layer with 5% sodium bicarbonate solution (50mL) and destroy monomethyl sulfate, wash and divide layer, then concentrate the organic layer to 165g, cool down at 0°C to crystallize for 2 hours, centrifuge, and dry t...
Embodiment 2
[0061] Dimethyl sulfate (13g; 0.2eq), methanol (105.6g; 6eq) and R-(+)-2-(4-hydroxyphenoxy)propionic acid (91g; 1eq) were added in a 500mL reaction flask, and the The reaction was carried out under reflux for 4-6 hours, and the esterification conversion rate monitored by HPLC was greater than 99.5%, and the reaction was completed. Lower the temperature, start to concentrate methanol under reduced pressure at 30°C, control the vacuum degree <-0.095MPa, concentrate at below 55°C, recover the methanol for use, add toluene (182g, twice the raw material) until it cannot evaporate, and cool down to 30-40°C , continue to distill methanol under reduced pressure, detect methanol<1% (GC: area normalization method), wash the sulfuric acid produced by the reaction in the toluene layer with 5% sodium bicarbonate solution (50mL) and destroy monomethyl sulfate, wash and divide Then concentrate the organic layer to 165g, cool down at 0°C for crystallization for 2 hours, centrifuge, and dry th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com