Aromatic hydrocarbon fluorine ortho-metallization preparation method
A metallization and ortho-position technology, which is applied in the preparation of organic compounds, halogenated hydrocarbons, carbon-based compounds, etc., can solve the problems of high cost of raw materials, high pressure of three wastes treatment, large amount of waste water, etc., and improve the conversion of raw materials low rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
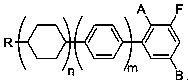
[0025] Example 1, a preparation method for the ortho-position metallation of arene fluorine, is to add the compound shown in formula I into an organic solvent, then add a catalytic amount of organic amine, mix well, cool down to below -50°C, and add lithium reagent dropwise , keep warm for 2-3 hours, carry out metallization reaction, after the reaction is completed, add electrophile reagent below -50°C, react with phenyllithium, keep warm for 2-3 hours, and obtain formula II after hydrolysis;
[0026]
[0027] I
[0028]
[0029] II
[0030] In formula Ⅰ, R is C1~C8 straight chain alkanes or C1~C8 straight chain alkoxyls, n=0,1,2, m=0,1, A is hydrogen atom or fluorine atom, B is hydrogen atom or fluorine Atoms, A and B are not fluorine atoms at the same time;
[0031] In formula II, R is C1~C8 straight chain alkane or C1~C8 straight chain alkoxyl, n=0,1,2, m=0,1, A is hydrogen atom or fluorine atom, B is hydrogen atom or fluorine Atoms, A and B are not fluorine atoms ...
Embodiment 2
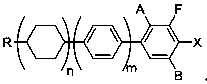
[0035] Example 2, a preparation method for the ortho-position metallation of arene fluorine, is to add the compound shown in formula I into an organic solvent, then add a catalytic amount of organic amine, mix well, cool down to below -50°C, and add lithium reagent dropwise , keep warm for 2-3 hours, carry out metallization reaction, after the reaction is completed, add electrophile reagent below -50°C, react with phenyllithium, keep warm for 2-3 hours, and obtain formula II after hydrolysis;
[0036]
[0037] I
[0038]
[0039] II
[0040] In formula Ⅰ, R is C1~C8 straight chain alkanes or C1~C8 straight chain alkoxyls, n=0,1,2, m=0,1, A is hydrogen atom or fluorine atom, B is hydrogen atom or fluorine Atoms, A and B are not fluorine atoms at the same time;
[0041] In formula II, R is C1~C8 straight chain alkane or C1~C8 straight chain alkoxyl, n=0,1,2, m=0,1, A is hydrogen atom or fluorine atom, B is hydrogen atom or fluorine Atoms, A and B are not fluorine atoms ...
Embodiment 3
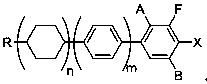
[0044] Example 3, a preparation method for the ortho-position metallation of arene fluorine, is to add the compound shown in formula I into an organic solvent, then add a catalytic amount of organic amine, mix well, cool down to below -50°C, and add lithium reagent dropwise , keep warm for 2-3 hours, carry out metallization reaction, after the reaction is completed, add electrophile reagent below -50°C, react with phenyllithium, keep warm for 2-3 hours, and obtain formula II after hydrolysis;
[0045]
[0046] I
[0047]
[0048] II
[0049] In formula Ⅰ, R is C1~C8 straight chain alkanes or C1~C8 straight chain alkoxyls, n=0,1,2, m=0,1, A is hydrogen atom or fluorine atom, B is hydrogen atom or fluorine Atoms, A and B are not fluorine atoms at the same time;
[0050] In formula II, R is C1~C8 straight chain alkane or C1~C8 straight chain alkoxyl, n=0,1,2, m=0,1, A is hydrogen atom or fluorine atom, B is hydrogen atom or fluorine Atoms, A and B are not fluorine atoms at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com