Method for detecting slow virus quality index combination and application of method
A quality index and lentivirus technology, which is applied in the titer detection field of lentiviral vectors, can solve the problems of many influencing factors, poor data repeatability, large sample demand, etc., and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: The method for detecting the total copy number of virus RNA by real-time fluorescent quantitative qPCR
[0057] The qPCR primers for detecting the total copy number of lentiviral RNA are SEQ ID No1-No 17, wherein the primers of SEQ ID No1-No9 are specific to the EF-1a promoter located in the vector plasmid, and the primers of SEQ ID No 10-No 13 are specific to the EF-1a promoter located in the vector plasmid The primers of WPRE, SEQ ID No14-17 are specific to the CD19-specific chimeric antigen receptor (CAR) part.
[0058] Specific steps are as follows:
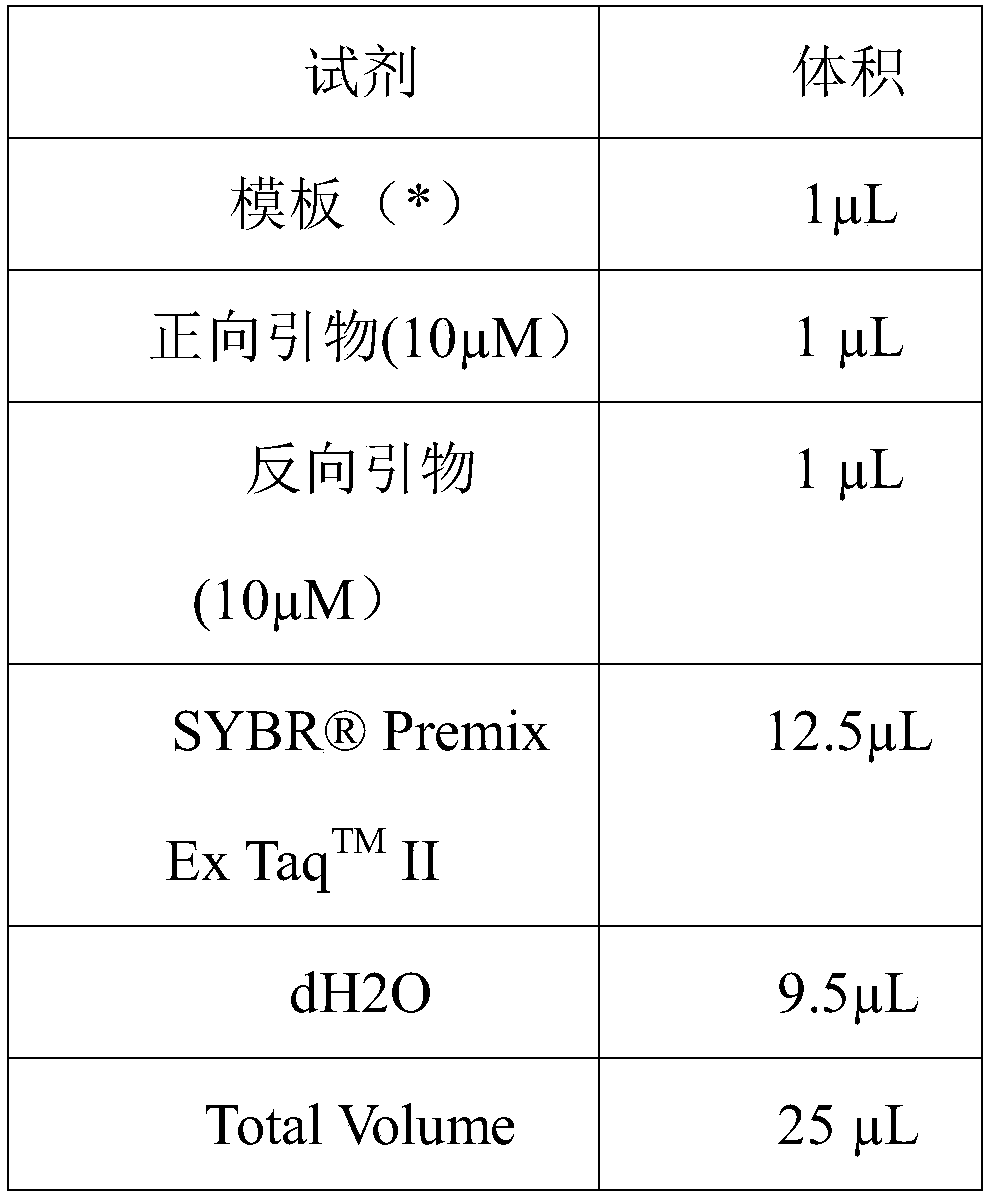
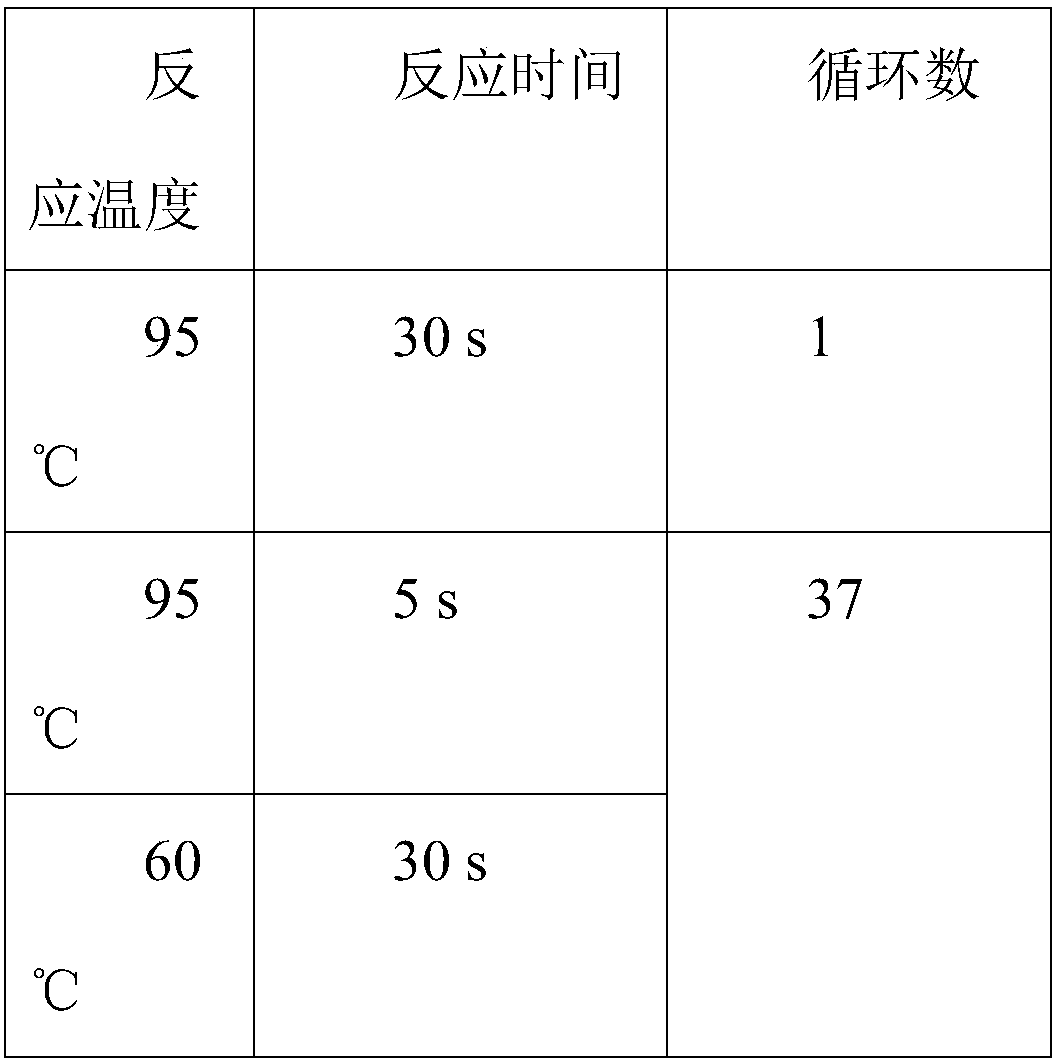
[0059] (1) Extraction of template DNA: the total DNA of the lentivirus sample to be tested was extracted with a viral RNA / DNA extraction kit (MiniBEST Viral RNADNA Extraction Kit from Takara). See the kit instructions for specific steps. DNA was diluted to 200ng / μL and 100ng / μL. (2) Reverse transcription reaction (RT): Using a reverse transcription kit (Takara PrimeScript RTMaster Mix (Perfect Real Tim...
Embodiment 2
[0077] Example 2: Method for detecting the copy number of virus integrated into chimeric antigen receptor T cells by real-time fluorescent quantitative qPCR
[0078] The qPCR primers for detecting the copy number of virus integrated into T cells are SEQ ID No18-No 23. The templates of the primers of SEQ ID No18-No21 are T cell genomes, which are determined by qPCR; the templates corresponding to the primers of SEQ ID No22-23 are T cell RNAs; RT-qPCR is to be performed.
[0079] Specific steps are as follows:
[0080] (1) Extraction of template DNA or RNA: Extract the DNA or RNA of the chimeric antigen receptor T cell sample to be tested with a viral RNA / DNA extraction kit (MiniBEST ViralRNA DNA Extraction Kit from Takara), and dilute each DNA or RNA sample to 200ng / μL and 100ng / μL.
[0081] (2) Gradient dilution of the standard: Calculate the initial standard copy number / ul of the vector plasmid according to the following formula:
[0082]
[0083] The standard for detec...
Embodiment 3
[0094] Example 3: Method for detecting the copy number of replication-competent lentivirus by real-time fluorescent quantitative qPCR
[0095] The qPCR primers for detecting the copy number of the replication-competent lentivirus are SEQ ID No24-No 27, and the primers are located at VSV-G of the envelope plasmid.
[0096] Specific steps are as follows:
[0097] (1) Extraction of template DNA: DNA of the sample to be tested was extracted with a viral RNA / DNA extraction kit (Takara's MiniBEST Viral RNADNA Extraction Kit). DNA was diluted to 200ng / ul and 100ng / ul.
[0098] (2) Gradient dilution of the standard: Calculate the copy number of the initial standard of the vector plasmid / μL according to the following formula
[0099]
[0100] Prepare a series of dilution standards with different copy numbers by serial dilution, each copy number is 10 7 、10 6 、10 5 、10 4 、10 3 、10 2 、10 1
[0101] The standard for measuring the copy number of replication-competent lentiviru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com