Preparation method and application of amino acid ester compound containing 4-piperidyl-6-methylpyrimidine heterocyclic ring
A technology of ester compounds and methyl pyrimidines, applied in the field of chemistry, can solve the problem of no ideal disease-resistant varieties, and achieve the effects of simple operation, high reaction yield, and easy availability of synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
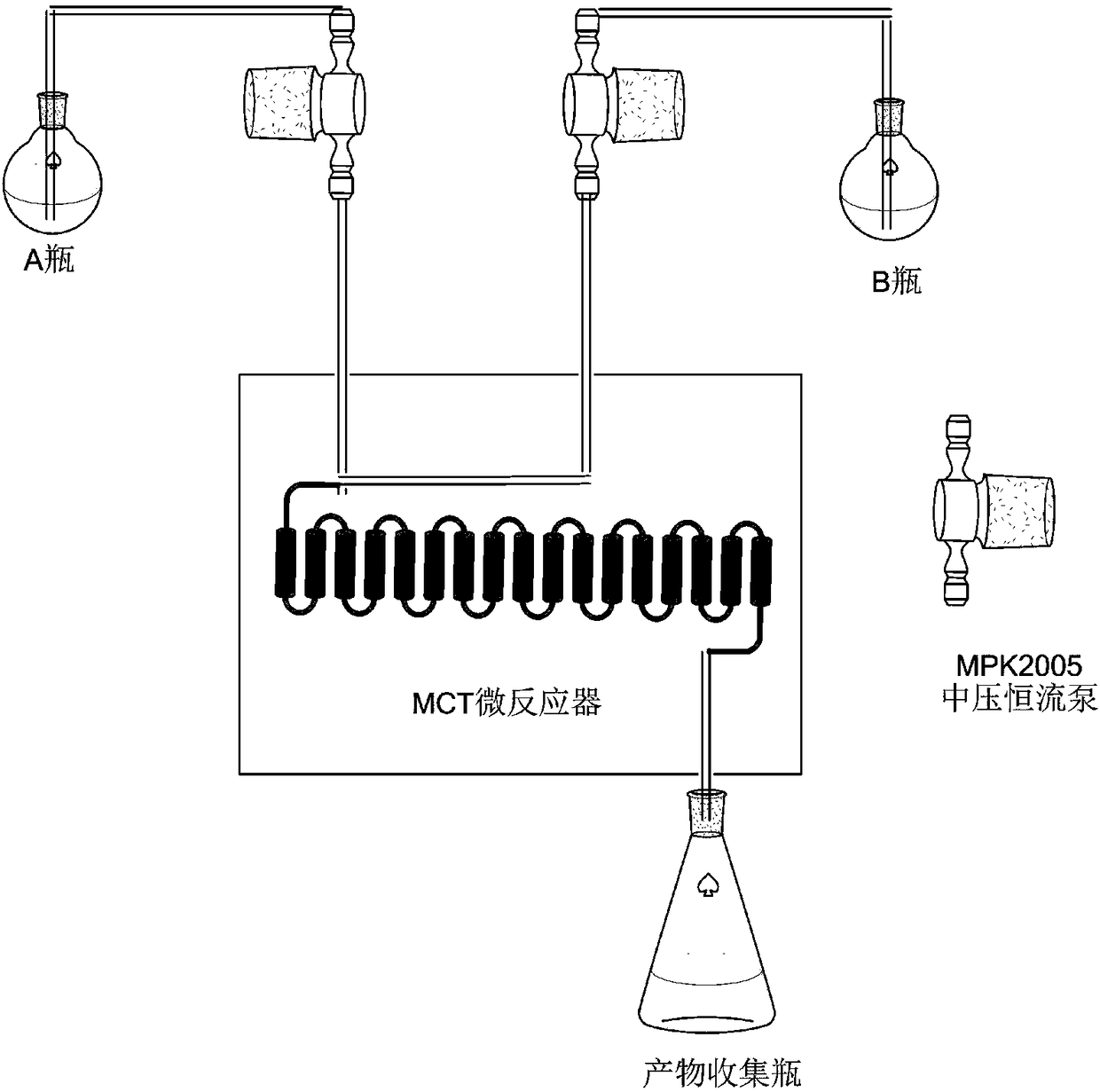
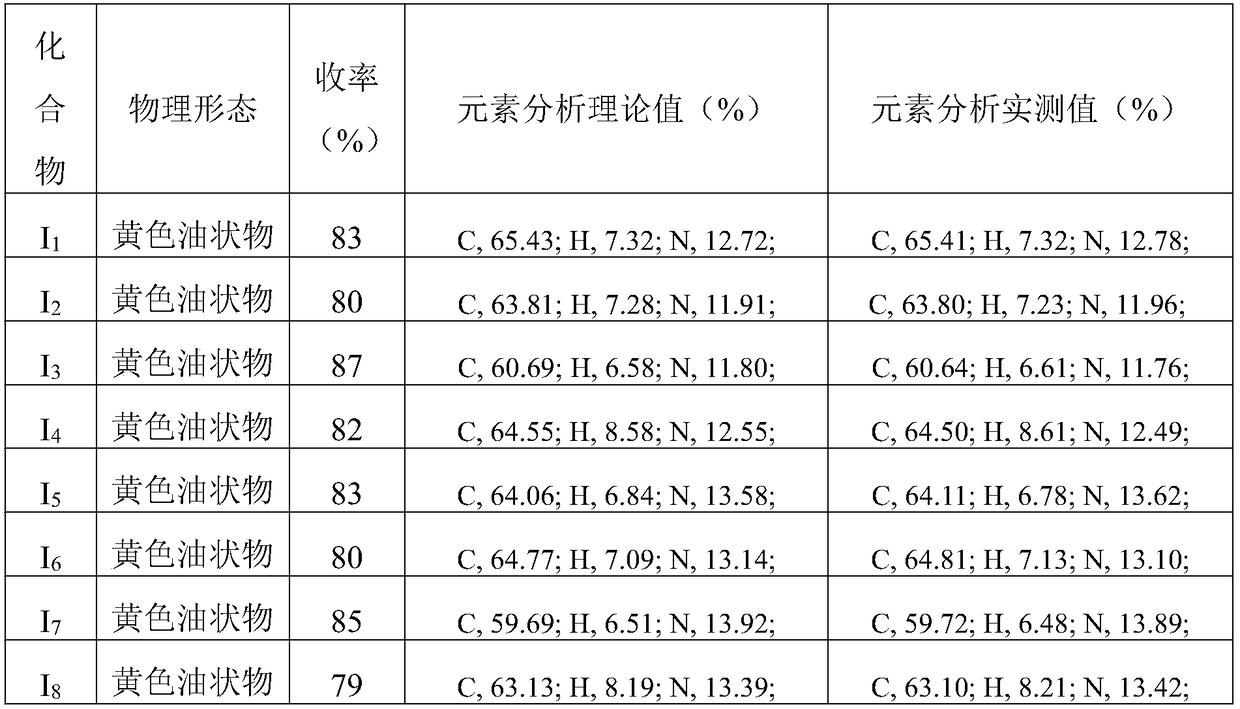
Image
Examples
Embodiment 1
[0040] Example 1: Compound I 1 Synthesis:
[0041] In a 100mL single-necked bottle, add 2-amino-4-piperidinyl-6-methylpyrimidine (0.001mol), benzaldehyde (0.001mol), diethyl malonate (0.0015mol), add p-xylene ( 30mL) as solvent, microwave at 100°C, finish the reaction after 50 minutes, recover p-xylene under reduced pressure, and separate by column chromatography (petroleum ether:ethyl acetate=4:1V / V) to obtain the target product.
Embodiment 2
[0042] Embodiment 2: Compound I 2 Synthesis:
[0043]In a 100mL single-necked bottle, add 2-amino-4-piperidinyl-6-methylpyrimidine (0.001mol), 4-methoxybenzaldehyde (0.001mol), diethyl malonate (0.0015mol), Add p-xylene (30mL) as a solvent, microwave at 100°C, stop the reaction after 50 minutes, recover p-xylene under reduced pressure, and separate by column chromatography (petroleum ether: ethyl acetate = 4:1 V / V) to obtain the target product.
Embodiment 3
[0044] Embodiment 3: Compound I 3 Synthesis:
[0045] In a 100mL single-necked bottle, add 2-amino-4-piperidinyl-6-methylpyrimidine (0.001mol), 4-chlorobenzaldehyde (0.001mol), diethyl malonate (0.0015mol), and add p Xylene (30 mL) was used as a solvent, and the reaction was terminated after 50 minutes under microwave at 100° C., p-xylene was recovered under reduced pressure, and the target product was obtained through column chromatography (petroleum ether: ethyl acetate = 4:1 V / V).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com