2-substituent-5-substituted anilino-1,3,4-oxadiazole derivatives and their synthesis method and application
An oxadiazole-based and methylaniline-based technology is applied in the directions of botanical equipment and methods, applications, and drug combinations, and can solve problems such as few therapeutic agents, unsatisfactory control effects, and pesticide residues.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
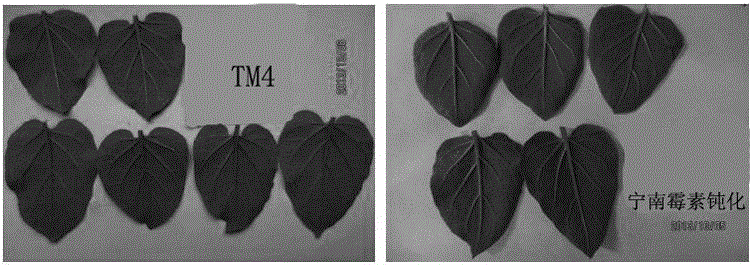
Image
Examples
Embodiment 1
[0093] Example 1: Preparation of 2-(methylthio)-5-anilino-1,3,4-oxadiazole (TM1)
[0094] (1) Preparation of ethyl anilinate intermediate:
[0095] Throw aniline (20 g, 0.21 mol), pyridine (17 g, 0.21 mol) and anhydrous dichloromethane (100 mL) into a 250 mL three-necked flask, and slowly add ethyl chloroformate (30 g, 0.27 mol) dropwise under ice cooling Afterwards, the reaction at room temperature was completed for 2 h. The reaction was quenched, washed with water, and desolvated under reduced pressure to obtain 35.1 g of ethyl anilinate with a yield of 86%.
[0096] (2) Preparation of anilinoformohydrazide intermediate
[0097] Throw ethyl anilinate (16 g, 0.18 mol) and 80% hydrazine hydrate (90 g, 1.80 mol) into a 100 mL three-necked round-bottomed flask, and heat up to reflux for 20 h to complete the reaction. After cooling, white crystals were precipitated, which were obtained by suction filtration to obtain anilinoformohydrazide, and recrystallized in absolute ethano...
Embodiment 2
[0103] Example 2: Preparation of 2-(4-methylbenzylthio)-5-anilino-1,3,4-oxadiazole (TM2)
[0104] Steps (1)-(4) are the same as in Embodiment 1.
[0105] (5) Add intermediate 2-mercapto-5-(anilino)-1,3,4-oxadiazole potassium salt (0.50g, 2.0 mmol), 15 mL water, potassium carbonate (0.28 g, 2.0 mmol), stirred, added (0.34 g, 2.5 mmol) iodomethane and KI (16.6 mg, 0.10 mmol). The reaction was stirred at room temperature (20 °C) for 5 h. The reaction solution was extracted with dichloromethane, concentrated, and separated by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain a white solid: 0.224 g, yield 35.5%.
Embodiment 3
[0106] Example 3: Preparation of 2-(4-fluorobenzylthio)-5-(2-methylanilino)-1,3,4-oxadiazole (TM3)
[0107] Steps (1)-(4) are the same as in Embodiment 1.
[0108] (5) Add intermediate 2-mercapto-5-(2-methylanilino)-1,3,4-oxadiazole potassium salt (0.80 g, 3.3 mmol), 15 mL water, Potassium carbonate (0.45 g, 3.3 mmol), stirred, added (0.77 g, 4.1 mmol) 4-fluorobenzyl bromide and KI (27.1 mg, 0.16 mmol). The reaction was stirred at room temperature (20 °C) for 5 h. The reaction solution was extracted with dichloromethane, concentrated, and separated by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain a white solid: 0.416 g, yield 40.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com