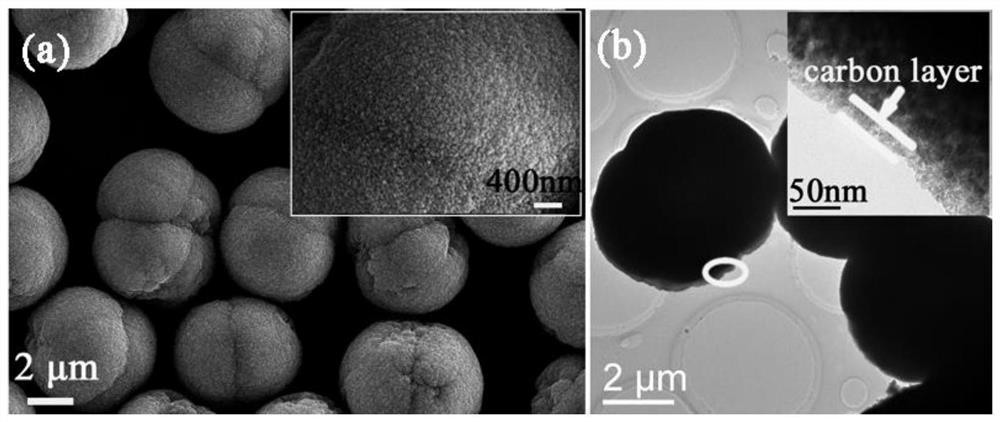
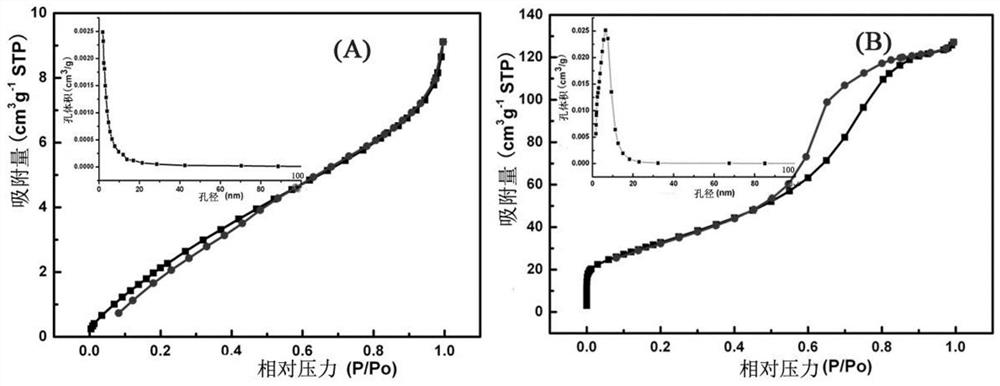
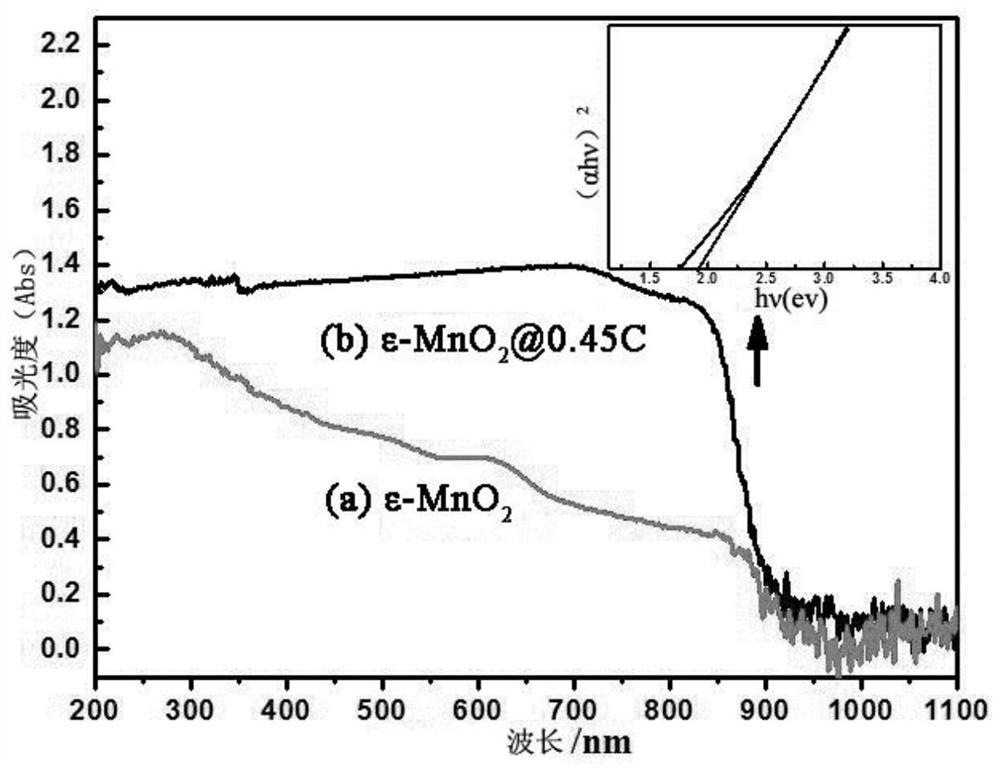
A kind of visible light response catalyst and its preparation method and application
A visible light and catalyst technology is applied in the field of photocatalytic materials to achieve the effects of strong adsorption capacity, strong visible light activity, and improved effective utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Mn(NO 3 ) 2 solution and 0.15g of glucose were added to ethylene glycol (50mL), magnetically stirred and mixed to obtain a mixed solution, wherein the final concentration of tetrabutylammonium bromide was 0.12mol / L, and the final concentration of urea was 0.34mol / L L, urea and Mn(NO 3 ) 2 The molar ratio is 0.6, and the final concentration of glucose is 0.01mol / L; transfer the mixed solution to a polytetrafluoroethylene-lined stainless steel autoclave, and conduct a hydrothermal reaction at 160°C in an electric constant temperature drying oven for 1 hour; After cooling to room temperature, the reaction product was taken out, and the reaction product was centrifuged to obtain a precipitate; the precipitate was washed three times with deionized water and absolute ethanol, and dried at 60°C for 12 hours to obtain an intermediate product;
[0042] (2) The intermediate product prepared in step (1) was calcined at 400°C for 3 hours in an air atmosphere to obtain a visi...
Embodiment 2
[0044] (1) Mn(NO 3 ) 2Solution and 0.25g of glucose were added in ethylene glycol (50mL), magnetically stirred and mixed to obtain a mixed solution, wherein the final concentration of tetrabutylammonium bromide was 0.12mol / L, and the final concentration of urea was 0.34mol / L L; transfer the mixed solution to a polytetrafluoroethylene-lined stainless steel autoclave, and conduct a hydrothermal reaction at 160°C in an electric constant temperature drying oven for 1 h; after the reaction kettle is naturally cooled to room temperature, the reaction product is taken out, and the reaction product is centrifuged to obtain Precipitate; the precipitate was washed three times with deionized water and absolute ethanol, and dried at 60°C for 12 hours to obtain an intermediate product;
[0045] (2) The intermediate product prepared in step (1) was calcined at 400°C for 3 hours in an air atmosphere to obtain a visible light-responsive catalyst (carbon-coated manganese dioxide ε-MnO 2 @0.2...
Embodiment 3
[0047] (1) Mn(NO 3 ) 2 solution and 0.45g of glucose were added to ethylene glycol (50mL), magnetically stirred and mixed to obtain a mixed solution, wherein the final concentration of tetrabutylammonium bromide was 0.12mol / L, and the final concentration of urea was 0.34mol / L L; transfer the mixed solution to a polytetrafluoroethylene-lined stainless steel autoclave, and conduct a hydrothermal reaction at 160°C in an electric constant temperature drying oven for 1 h; after the reaction kettle is naturally cooled to room temperature, the reaction product is taken out, and the reaction product is centrifuged to obtain Precipitate; the precipitate was washed three times with deionized water and absolute ethanol, and dried at 60°C for 12 hours to obtain an intermediate product;
[0048] (2) The intermediate product obtained in step (1) was calcined at 400° C. for 3 h in an air atmosphere to obtain a visible light-responsive catalyst (carbon-coated manganese dioxide ε-MnO2@0.45C)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com