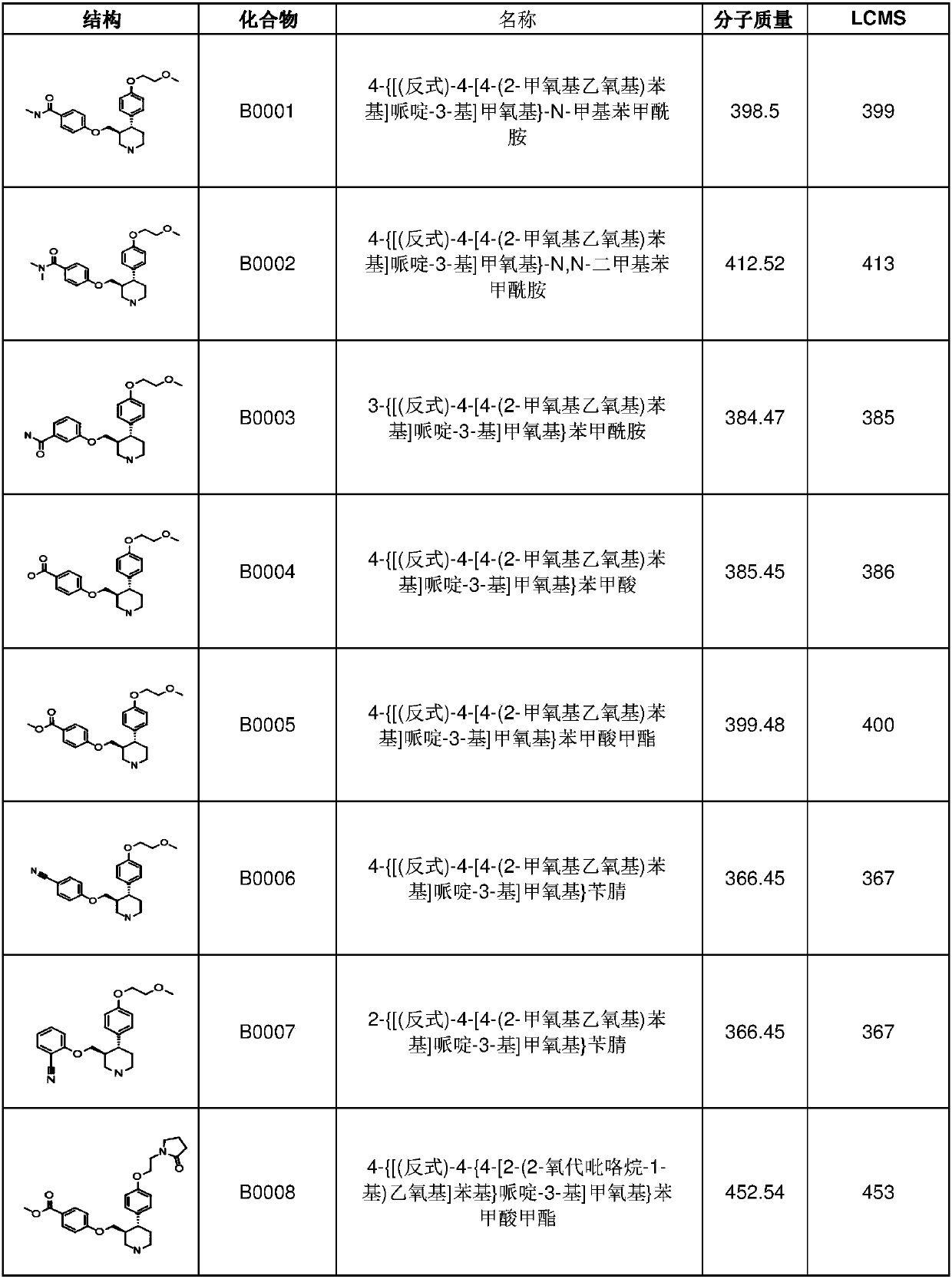
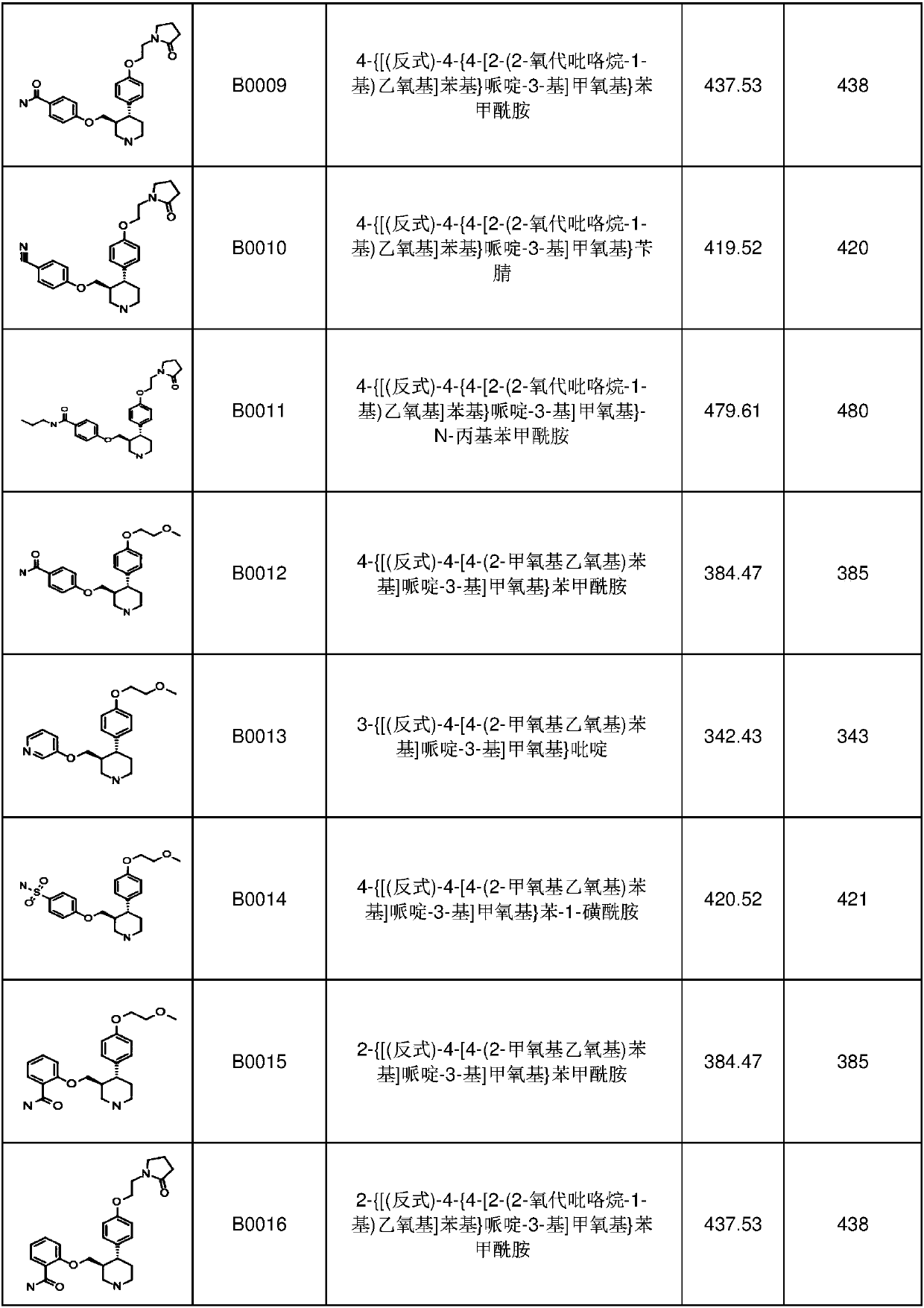
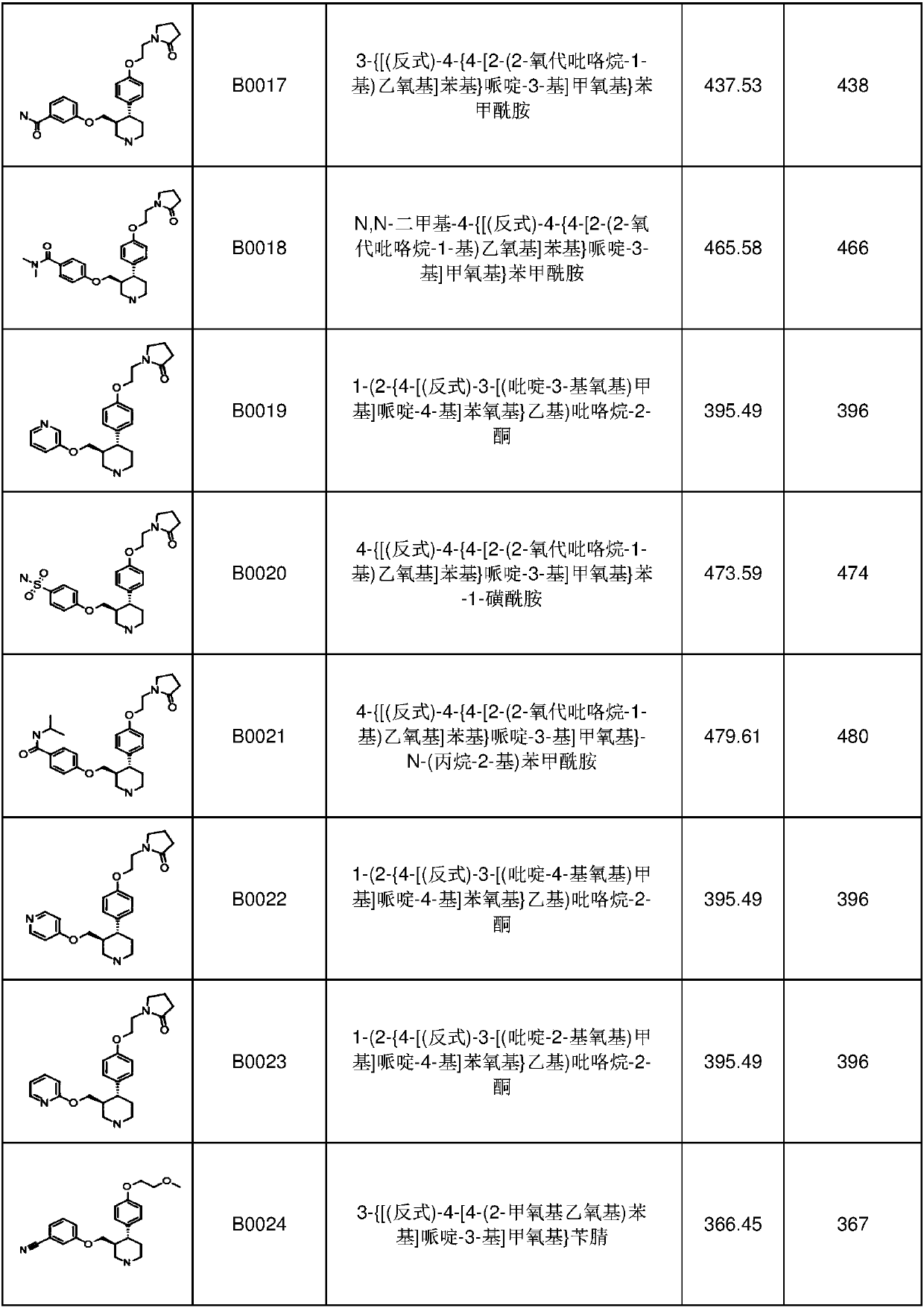
6-membered aza-heterocyclic containing delta-opioid receptor modulating compounds, methods of using and making the same
A compound and heterocycle technology, applied in the field of delta opioid receptor modulating compounds containing six-membered aza heterocycles, their use and preparation, and can solve problems such as hindering the development of selective drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0347] 1. A compound having formula I, I-1, I-a, I-a1 or I-b, Ib-1 or Ib-2
[0348]
[0349]
[0350]
[0351] or a pharmaceutically acceptable salt thereof, wherein:
[0352] BB is
[0353] Z is C, S, N, S(O) 2 or O;
[0354] R 35 is a protecting group, C(=O)OR 81b , H, optionally substituted aryl, optionally substituted C 1 -C 6 Haloalkyl, -R 63 R 64 , optionally substituted C 1 -C 6 Branched or unbranched alkyl, optionally substituted C 2 -C 6 Alkenyl, optionally substituted C 2 -C 6 Haloalkenyl-(CH 2 ) n R 65 , optionally substituted heterocycle, optionally substituted C 1 -C 6 Esters, optionally substituted cycloalkyl, optionally substituted C 1 -C 6 Alkoxy, optionally substituted pyrrolinyl, optionally substituted morpholinyl, optionally substituted C 3 -C 6 Cyclic ether or optionally substituted piperidinyl;
[0355] R 36 is empty, H, halogen, optionally substituted C 1 -C 6 Haloalkyl, -SO 2 C 1 -C 6 Alkyl, -OCF 3 , optionally su...
Embodiment 1
[0711] Example 1:
[0712] General Procedure A1: Preparation of 3,4-Piperidine N-H Analogs
[0713] plan 1
[0714]
[0715] Scenario 2
[0716]
[0717] (+)-6-{[trans-4-(4-methoxyphenyl)piperidin-3-yl]methoxy}isoindolin-1-one[(+)B0515] and (- )-6-{[trans-4-(4-methoxyphenyl)piperidin-3-yl]methoxy}isoindolin-1-one[(-)B0523] (Scheme 1 )
[0718] 4-(Trifluoromethylsulfonyloxy)-5,6-dihydropyridine-1,3(2H)-dicarboxylic acid 1-tert-butyl 3-ethyl ester (2)
[0719] Trifluoromethanesulfonic anhydride (33.3 mL, 203 mmol) was added portionwise to commercially available (+ / -) -4-oxopiperidine-1,3-dicarboxylic acid 1-tert-butyl 3-ethyl ester ([+ / -]1, 50.0g, 184mmol] and N,N-diisopropylethylamine (48.3 mL, 277 mmol). The mixture was stirred at 0 °C for 2 h, then warmed to room temperature, and the solids were removed by filtration. The filtrate solvent was removed under reduced pressure to afford crude 2 as a brown oil, which was used in the next step without purification St...
Embodiment 2
[1190] Example 2: Compounds are effective as modulators of delta-opioid receptors
[1191] The compounds described herein were tested as modulators of delta-opioid receptors. These compounds were found to be modulators of receptor activity. Some compounds inhibit the β-arrestin pathway and the G protein-mediated pathway, while other compounds agonize or enhance the β-arrestin-mediated pathway or the G-protein-mediated pathway. Activity was measured according to the methods described herein. The compounds described herein were tested as modulators of mu and kappa-opioid receptors.
[1193] Plasmids encoding the δ opioid receptor (accession number NP_000902), the μ opioid receptor (accession number NP_000905) and the kappa opioid receptor (accession number NP_000903) were generated in the pCMV-Prolink backbone and transfected into EA-arrestin parental human embryonic kidney (HEK-293) cell line. Clonal stable lines were subsequently selected under G41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com