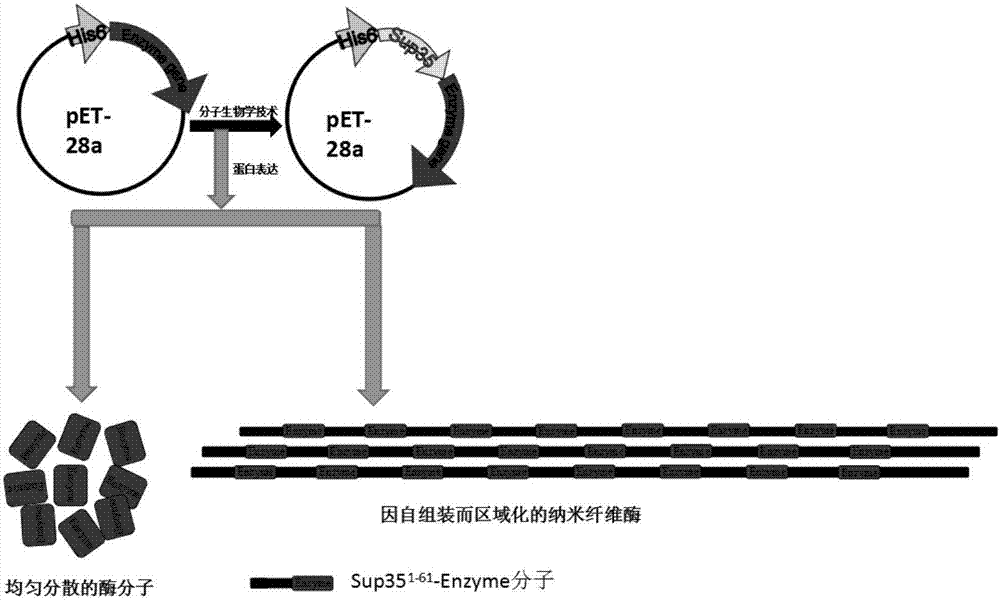
Enzyme compound and self-assembled catalytic nanowire
A complex and self-assembly technology, applied in the direction of enzyme stabilization, can solve the problems of high cost, complex preparation, limited catalytic properties and stability, and achieve the effect of realizing length and control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1Sup35-MPH enzyme complex
[0077] 1. Cloning of Sup35-MPH enzyme complex
[0078] Yeast prion protein self-assembly domain (the 1-61st amino acid of Sup35, referred to as Sup35, the specific sequence is shown in SEQ ID NO.1) and methyl parathion hydrolase (the 36th-331th amino acid of MPH) were combined by molecular cloning amino acids, the specific sequence shown in SEQ ID NO.2) is fused and connected through a flexible linker peptide (see the specific sequence shown in SEQ ID NO.3) to form a fusion protein Sup35-MPH, and then a His tag is fused at its N-terminus. Methyl parathion hydrolase (MPH) alone was used as a control. Specific steps are as follows:
[0079] 1) Using the plasmid pET28a as a template, using the primer MPH-Primer-1 to carry out PCR amplification to obtain the plasmid pET28a containing the sequence of the primer MPH-Primer-1;
[0080] 2) using the MPH gene fragment as a template, using the primer MPH-Primer-3 to per...
Embodiment 2
[0090] Example 2 Detection of Sup35-MPH enzyme complexes and nanowires
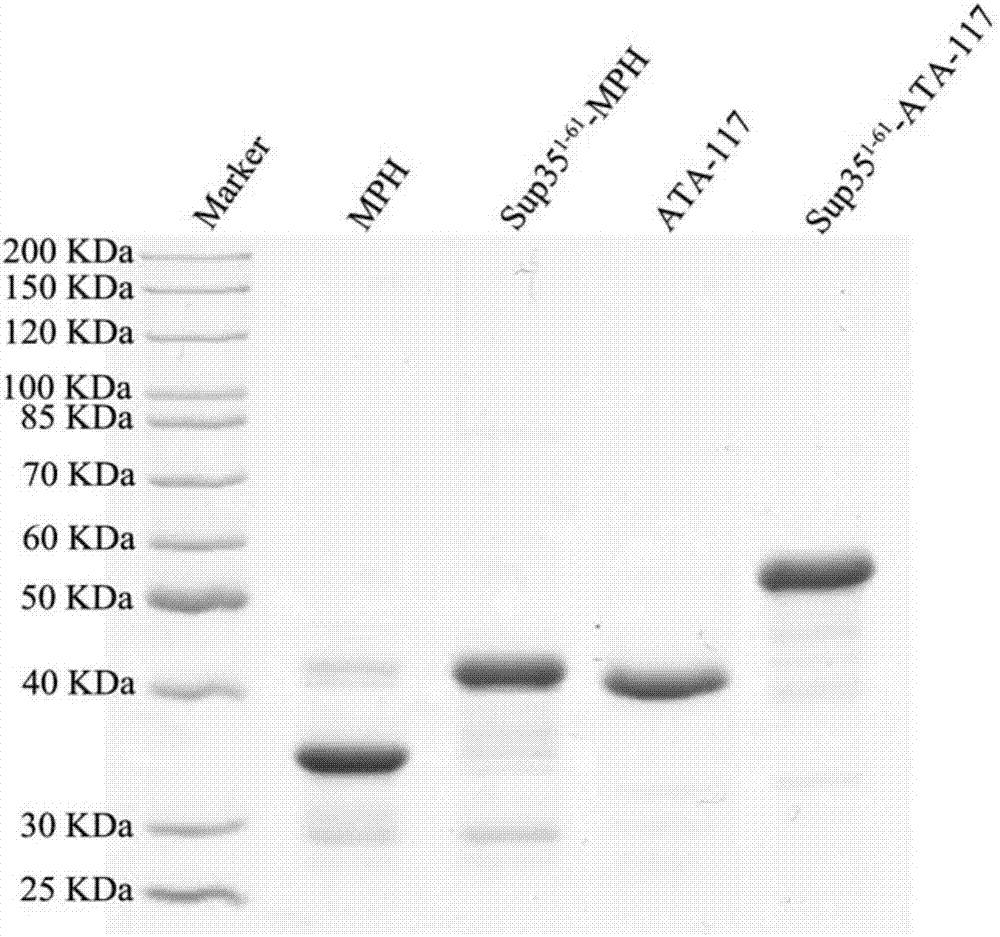
[0091] SDS-PAGE electrophoresis is carried out to the MPH and Sup35-MPH that embodiment 1 makes, the gel that obtains is carried out Coomassie brilliant blue staining, and its result is as follows figure 2 shown. Depend on figure 2 It can be seen that between 30KDa and 40KDa, there are clear and obvious bands around 40KDa, which correspond to free methyl parathion hydrolase protein (MPH) and Sup35-MPH enzyme complex respectively. Electrophoresis showed no obvious bands of miscellaneous proteins, indicating that the purity and expression level of the methyl parathion hydrolase protein (MPH) and Sup35-MPH enzyme complex prepared in Example 1 were high.
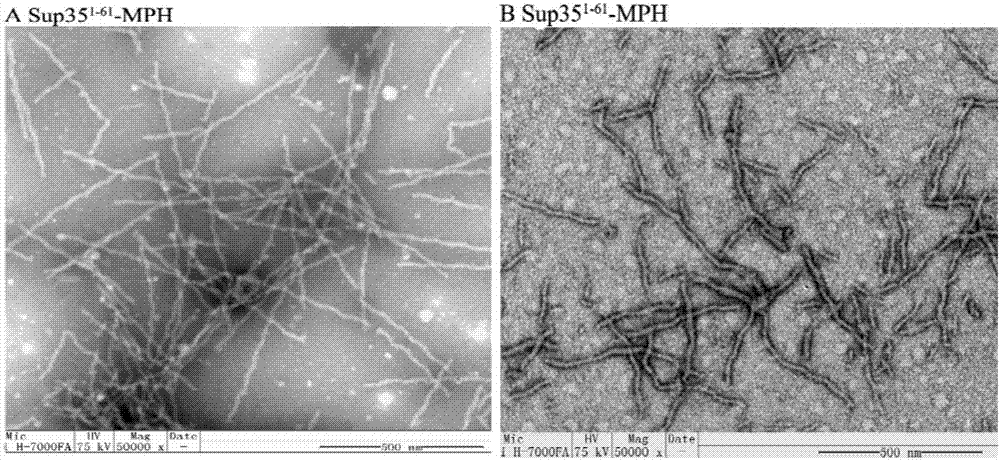
[0092] The self-assembled catalytic nanowire Sup35-MPH prepared in Example 1 was detected by electron microscopy, and the results were as follows image 3 As shown in A. Depend on image 3 A shows that the concentration and length distribution of nan...
Embodiment 3
[0093] The preparation of embodiment 3 Sup35-ATA-117 enzyme complex
[0094] 1. Cloning of Sup35-ATA-117 enzyme complex
[0095] Yeast prion protein self-assembly domain (1-61 amino acids of Sup35, referred to as Sup35, specific sequence is shown in SEQ ID NO.1) and sitagliptin aminotransferase (ATA-117, specific sequence is shown in SEQ ID NO.1) by molecular cloning Shown in NO.4) is fused and connected through a flexible linker peptide (see SEQ ID NO.3 for the specific sequence) to form a fusion protein Sup35-ATA-117, and then a His tag is fused to its N-terminus. Sitagliptin aminotransferase (ATA-117) alone was used as a control. Specific steps are as follows:
[0096] 1) Using the plasmid pET28a as a template, using the primer ATA-Primer-1 to carry out PCR amplification to obtain the pET28a vector containing the sequence of the primer ATA-Primer-1;
[0097] 2) Use the plasmid PUC57-ATA-117 as a template, and use the primer ATA-Primer-2 to perform PCR amplification to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com