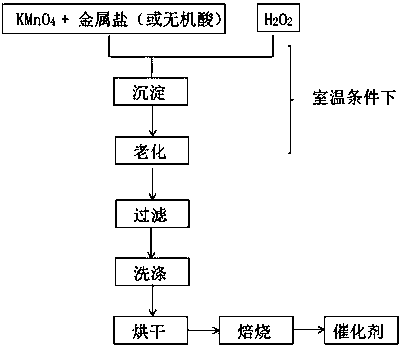
Preparation of bimetallic oxides by redox-hydrolysis coupling reaction for low-temperature catalytic combustion of vocs
A bimetallic oxide, low-temperature catalysis technology, applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc. sufficient, waste water and slag, etc., to achieve the effect of favorable adsorption, large specific surface area, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 6 g of KMnO 4 and 5.1 g Fe(NO 3 ) 3 9H 2 O was dissolved in 200-300 mL of deionized water to form a deep purple aqueous solution 1, in which the molar ratio of Mn and Fe was 3:1;
[0033] (2) 19.35mL of analytically pure (30%) H 2 o 2 Dilute with 200-300 mL deionized water to obtain solution 2;
[0034] (3) At room temperature with rapid stirring, solution 2 was added dropwise to solution 1, and a large amount of precipitate was formed during the reaction, accompanied by a large amount of O 2 generate;
[0035] (4) Leave the precipitate to age overnight, filter and wash with water for 3-5 times;
[0036] (5) at 110 o C drying overnight, roasting in air to 400 o C and kept for 2 h, the 3Mn1Fe double metal oxide catalyst with a Mn / Fe molar ratio of 3:1 can be obtained;
[0037] (6) Characterized by scanning electron microscopy, the 3Mn1Fe synthesized in Example 1 is amorphous nanoparticles on the microscopic scale, see figure 2 ; Characterized by trans...
Embodiment 2
[0040] (1) Add 6 g of KMnO 4 , 3.1 g of Fe(NO 3 ) 3 9H 2 O and 1.48 g of concentrated nitric acid were dissolved in 200-300 mL of deionized water to form a deep purple aqueous solution 1, wherein the molar ratio of Mn and Fe was 5:1;
[0041] (2) 19.35 mL of analytically pure (30%) H 2 o 2 Dilute with 200-300 mL deionized water to obtain solution 2;
[0042] (3) At room temperature with rapid stirring, solution 2 was added dropwise to solution 1 to form a large amount of precipitate accompanied by a large amount of O 2 generate;
[0043] (4) Leave the precipitate to age overnight, filter and wash with water for 3-5 times;
[0044] (5) at 110 o C drying overnight, roasting in air to 400 o C and keep it for 2 h, you can get 5Mn1Fe double metal oxide;
[0045] (6) Characterized by scanning electron microscopy, the 5Mn1Fe double metal oxide synthesized in Example 2 is amorphous nanoparticles on the microscopic scale, see Figure 4 ; characterized by XRD as amorphous str...
Embodiment 3
[0048] (1) Add 6 g of KMnO 4 and 5.52 g of Co(NO 3 ) 2 ·6H 2 O was dissolved in 200-300 mL of deionized water to form a deep purple aqueous solution 1, in which the molar ratio of Mn and Co was 2:1;
[0049] (2) 19.35 mL of analytically pure (30%) H 2 o 2 Dilute with 200-300 mL deionized water to obtain solution 2;
[0050] (3) At room temperature with rapid stirring, solution 2 was added dropwise to solution 1 to form a large amount of precipitate accompanied by a large amount of O 2 generate;
[0051] (4) Leave the precipitate to age overnight, filter and wash with water for 3-5 times;
[0052] (5) at 110 o C drying overnight, roasting in air to 400 o C and keep it for 2 h, you can get 2Mn1Co double metal oxide;
[0053] (6) Characterized by XRD, the catalyst is an amorphous oxide;
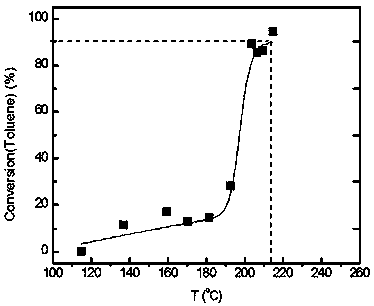
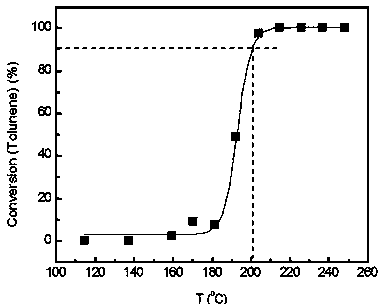
[0054] (7) In a fixed-bed reactor, evaluate the catalytic combustion performance of high-concentration toluene. The catalyst dosage is 0.2 g. The catalyst is 40-60 mesh solid particle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com