Ulipristal acetate dispersible tablet and preparation method thereof
A technology of ulipristal acetate and dispersible tablets, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of low affinity of estrogen and mineralocorticoid receptors, etc. The effect of high Lilistat content, fast disintegration and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
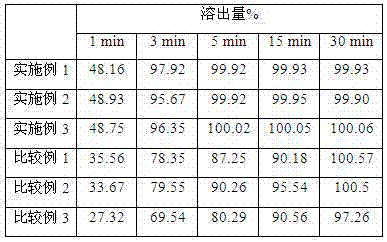
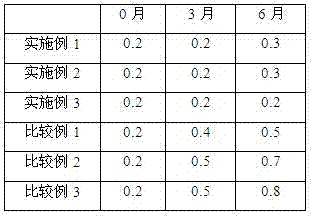
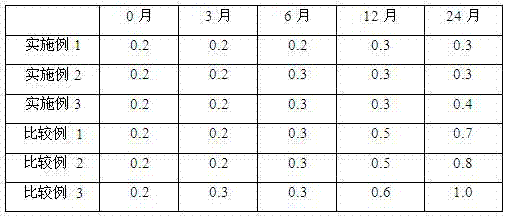
Examples
Embodiment 1
[0032] The main ingredients and excipients of Ulipristal Acetate Dispersible Tablets (1000 tablets) are as follows:
[0033] Ulipristal acetate 30 g (10%)
[0034] Lactose 166 g (55.33%)
[0035] Mannitol 50 g (16.67%)
[0036] Povidone K30 30 g (10%)
[0037] Dioctyl sodium sulfosuccinate 6 g (2%)
[0038] Croscarmellose Sodium 15 g (5%)
[0039] Magnesium stearate 3 g (1%).
[0040] The preparation method of above-mentioned ulipristal acetate dispersible tablet is as follows:
[0041] (1) Weigh the main ingredients and auxiliary materials according to the weight percentage, and pass through 80-mesh sieve respectively, and set aside;
[0042] (2) Povidone K30 is dissolved in an ethanol solution with a mass fraction of 75% to obtain a povidone solution, and the povidone mass fraction in the povidone solution is 20%; dioctyl sulfosuccinate Add sodium and ulipristal acetate to the povidone solution and mix well;
[0043] (3) Mix lactose, mannitol and croscarmellose sodiu...
Embodiment 2
[0047] The main ingredients and excipients of Ulipristal Acetate Dispersible Tablets (1000 tablets) are as follows:
[0048] Ulipristal acetate 30 g (10%)
[0049] Lactose 155 g (51.67%)
[0050] Microcrystalline Cellulose 46 g (15.33%)
[0051] Povidone K30 45 g (15%)
[0052] Sodium dodecylbenzenesulfonate 6 g (2%)
[0053] Crospovidone 15 g (5%)
[0054] Glyceryl Behenate 3 g (1%).
[0055] The preparation method of above-mentioned ulipristal acetate dispersible tablet is as follows:
[0056] (1) Weigh the main ingredients and auxiliary materials according to the weight percentage, and pass through 80-mesh sieve respectively, and set aside;
[0057] (2) Povidone K30 is dissolved in an ethanol solution with a mass fraction of 80% to obtain a povidone solution, and the mass fraction of povidone in the povidone solution is 30%; sodium lauryl sulfate and acetic acid Ulipristal is added to the povidone solution and mixed;
[0058] (3) Mix lactose, microcrystalline cellul...
Embodiment 3
[0062] The main ingredients and excipients of Ulipristal Acetate Dispersible Tablets (1000 tablets) are as follows:
[0063] Ulipristal acetate 30 g (10%)
[0064] Mannitol 136 g (45.33%)
[0065] Macrogol 6000 75 g (25%)
[0066] Povidone K30 30 g (10%)
[0067] Tween 80 9 g (3%)
[0068] Low-substituted hydroxypropyl cellulose 15 g (5%)
[0069] Stearic acid 3 g (1%)
[0070] Colloidal silicon dioxide 2 g (0.67%).
[0071] The preparation method of above-mentioned ulipristal acetate dispersible tablet is as follows:
[0072] (1) Weigh the main ingredients and auxiliary materials according to the weight percentage, and pass through 80-mesh sieve respectively, and set aside;
[0073] (2) Povidone K30 was dissolved in an ethanol solution with a mass fraction of 75% to obtain a povidone solution, and the mass fraction of povidone in the povidone solution was 30%; He added to povidone solution and mixed well;
[0074] (3) Mix mannitol, polyethylene glycol 6000, and low-sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com